Prying Apart the Mighty Bite of a Malaysian Trap-Jaw Ant
Its mandibles strike in a fraction of a blink of an eye, but how does it do it?
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/13/34/1334b320-47ca-4fa5-a69c-98e360ee1960/antphoto1.jpg)
Imagine you’re crawling along the forest floor, idly searching for a bit of fungus to chow down on, when out of nowhere appears an ant with bulging eyes and a pair of long, slender, razor blade-equipped mandibles drawn back behind its head. You attempt to hop away to safety, but the creature’s jaws are too quick—whipping around in half a millisecond, they impale you from two sides at once before you’ve gone anywhere at all. Such is a typical experience in the sad, short life of a springtail, prey of choice for the savage “trap-jaw” ants of the Myrmoteras genus.
Trap-jaw ants have long been a source of fascination for Fred Larabee, a postdoctoral researcher at the Smithsonian's National Museum of Natural History and lead author of a cutting-edge paper on the physiology of Myrmoteras specimens published today in the Journal of Experimental Biology. In the study, Larabee and his cohorts aim to answer two distinct questions about these rare Malaysian insects: exactly how fast are their lethal jaws, and how is it that they generate their power?
Myrmoteras—from the Greek for “monstrous ant”—is but one variety of trap-jaw ants, and an uncommon variety at that. Collecting four whole colonies for the study, two from each of two species within the genus, required extensive rummaging through Bornean jungle leaf litter. What makes trap-jaw research so fascinating to Larabee and other myrmecologists (ant biologists) is the functional similarity observed between species that evolved completely independent of one another.
“Trap-jaws are really remarkable,” says Larabee, who notes that they have developed in five distinct ant genera in five distinct forms. “They’ve evolved multiple times within ants. Being able to look at a completely different lineage, a different origin of the behavior and morphology, gives you a unique opportunity to study convergent evolution—basically the repeated, parallel evolution of this [trap-jaw] system.”
When offered the chance to work with Myrmoteras—a genus about which precious little was known—Larabee was over the moon. He had worked with the more common trap-jaw genera Anochetus and Odontomachus before, but knowing the nature of convergent evolution, he thought it plausible that the Myrmoteras ants had developed the same vicious attack capability via an entirely different anatomical means.
Larabee and his co-authors were expecting the Myrmoteras mandible attack to be unique, but the extent of its dissimilarity to those of other genera came as a surprise.
In order to measure the angular velocity of the ants’ crippling jaw strike, the team relied on high-speed photography.
“We used a camera that could film at 50,000 frames per second to slow down the motion,” he says, “and that was fast enough to be able to slow it down to actually measure the duration of a strike, and also the peak speed.”
At their fastest, the mandibles move at a linear speed of 60 miles per hour, and the whole of their motion is complete within about 1/700th of the time it takes a human to blink their eyes.
Amusingly though, what took Larabee by surprise was that this result wasn’t all that fast. “Compared to other trap-jaw ants, it’s pretty slow,” he says with a laugh. Indeed, the pincer motion of Odontomachus ants is fully twice as rapid.
Larabee supposed that the reason for the comparative sluggishness of Myrmoteras jaw strikes must have to do with the anatomical structures enabling them—the subject of the second part of his research.
In addition to the tried-and-true method of examining specimens under a microscope for clues as to the operation of their trap-jaw system, Larabee’s team brought to bear a modern technology previously untested in the realm of trap-jaw ant research: the X-ray micro-CT scan.
Essentially a shrunken-down version of the CAT scan you might receive at the doctor’s office, the micro-CT technique enables researchers like Larabee to get a better idea of the internal structures present in a given specimen, and how they are arranged in three-dimensional space.
“In a digital environment,” Larabee says, he was able to “look at the structures and see how they relate to each other, and where muscles are attaching to the mandible.” He is a huge proponent of the micro-CT technology, which provides significant insights without doing harm to the specimen. (Given that best practice for studying archived specimens is not to alter them, micro-CT could prove to be a major boon for Larabee's museum colleagues going forward.)
Evolutionary biologist and entomologist Corrie Moreau, a professor at Chicago’s Field Museum of Natural History, is excited by the technical rigor of the Myrmoteras research, and by its possible implications for the field.
“The real strength of this study by Larabee, Gronenberg, and Suarez,” she says, “is the diversity of tools and techniques the authors used to fully understand the mechanisms employed by this group of ants to achieve power amplification.”
What Larabee found with his CT analysis was that the lock, spring and trigger mechanisms that allow Myrmoteras to execute its jaw attacks were all likely significantly different from their equivalents in trap-jaw ants of other genera.
Most intriguing, perhaps, is the locking mechanism that keeps the jaws apart when not engaged. Prior to an assault, Myrmoteras mandibles are separated by an incredible 270 degrees—in Anochetus and Odontomachus, this angle is only 180. Micro-CT imaging shed some (high-energy) light on this, suggesting that “the opposing levers of two muscles pulling on the mandible favor the mandible staying open, because of the way the muscles are attached to the mandible joint.”
The Myrmoteras configuration is a bizarre one. “That’s a lock system you don’t see in other trap-jaw ants,” Larabee says.
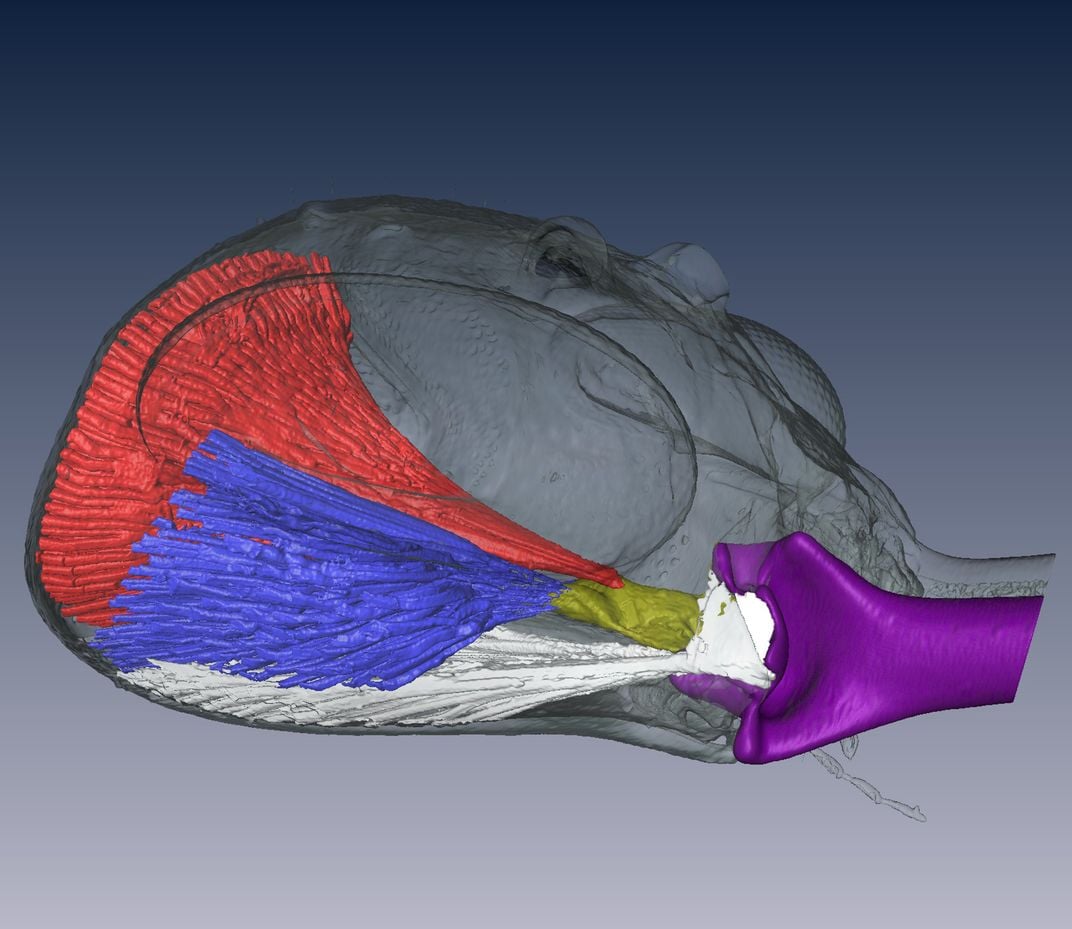
This unusual locking method informs another aspect of the jaw attack apparatus: the trigger. In the heads of other trap-jaw ants, the trigger muscle—which provides the mandibles with their initial torque—tends to be small. Because of the way the locking system functions in Myrmoteras, though, this trigger is significantly beefier, and is easily discerned in the CT scans.
Last but not least is the spring mechanism that allows Myrmoteras ants to store the potential energy that becomes kinetic energy when they let loose. Larabee hypothesizes that a primary source of this spring potential is a lobe at the back of the ants’ heads, which in the high-speed photography was seen to deform significantly during attacks. Additional research is required, but Larabee says the “deformation of the head is so big that we suspect that that’s got to contribute to the energy storage.”
All of these various factors come together to produce a single Myrmoteras strike, similar to the strikes of other far-flung trap-jaw genera at the macro level yet utterly idiosyncratic at the micro level. And while Myrmoteras attacks don’t pack quite as large a wallop as those of other ants, Larabee is quick to point out that they get the job done.
“Half a millisecond is nothing to sneeze at in terms of speed,” he says, “and it’s plenty fast to capture a springtail.” Even with their weaker apparatus, Myrmoteras ants generate about 100 times as much power with the elastic tools they have evolved than they ever could through direct muscle action alone.
Why exactly these ants developed this capability is unclear, but Larabee thinks it has a lot to do with their nimble targets. “You end up with these arms races between predators and prey,” he says. “If you're a gazelle, you have to run fast, and that means the cheetah's going to run even faster. And I suspect that having prey that are able to escape very quickly”—like springtails—“is a good pressure to select for these really fast predators.”
Moreau is optimistic that this research will open the door for further inquiries into the larger, often-astonishing world of convergent evolution.
“With so many ants, and other organisms, relying on power amplification to capture prey,” she says, one wonders, “How many ways can this effective strategy evolve across the animal kingdom? And this study nicely adds to our understanding of this very interesting question.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/DSC_02399_copy.jpg)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/DSC_02399_copy.jpg)