A Cool New Way to Freeze and Unfreeze Zebrafish Embryos Using Gold Nanotechnology and Lasers
The downstream applications could make food cheaper, repair coral reefs and help restore frog populations
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/97/9d/979d9257-273c-43f1-8505-220ed060b82f/zebrafish_26436913602.jpg)
For more than 20 years, marine biologist Mary Hagedorn faced a seemingly intractable problem. She was looking for a way to freeze and defrost the embryos of zebrafish.
An important experimental animal, zebrafish genes approximate those of humans closely enough that they’ve been used to investigate diseases such as muscular dystrophy and melanoma. If the reproductive material could be readily frozen and defrosted, those studies would be easier to conduct and replicate, since researchers wouldn’t have to work around spawning schedules or struggle against genetic drift.
The trouble comes down to the way fish reproduce. Scientists have been successfully freezing—or cryopreserving, to use the technical term—and defrosting viable sperm and eggs from many animals for decades. But fish eggs develop outside the parent’s body, which presents physiological challenges that don’t come up when you’re working with cells from cattle, or even humans. The egg contains the nutrients that the developing embryo will need and also has its own armor, meaning that those eggs are big and often encased in a relatively impermeable membrane.
To put it simply, the fish eggs tend to be too big to freeze or defrost quickly under ordinary circumstances. Hagedorn—who works as a research biologist with the Smithsonian’s National Zoo and Conservation Biology Institute’s Center for Species Survival—compares them to planets. Mammalian eggs are typically more like the tinier members of our solar system—say, Mercury. A zebrafish egg is closer to a giant like Jupiter.
“If you don’t freeze tissue properly, ice crystals will form in it and they’ll pierce the cells and destroy them,” Hagedorn says.
She spent 12 years in search of a workaround, ultimately settling on a novel solution that involved microinjecting a “cryoprotectant” (an antifreeze, basically) into the eggs, a technique that allowed that agent to bypass the protective membrane. Properly calibrated to avoid poisoning the cells, those protectants could help ensure that an egg would evenly vitrify (becoming glasslike) when it was dunked into a liquid nitrogen bath.
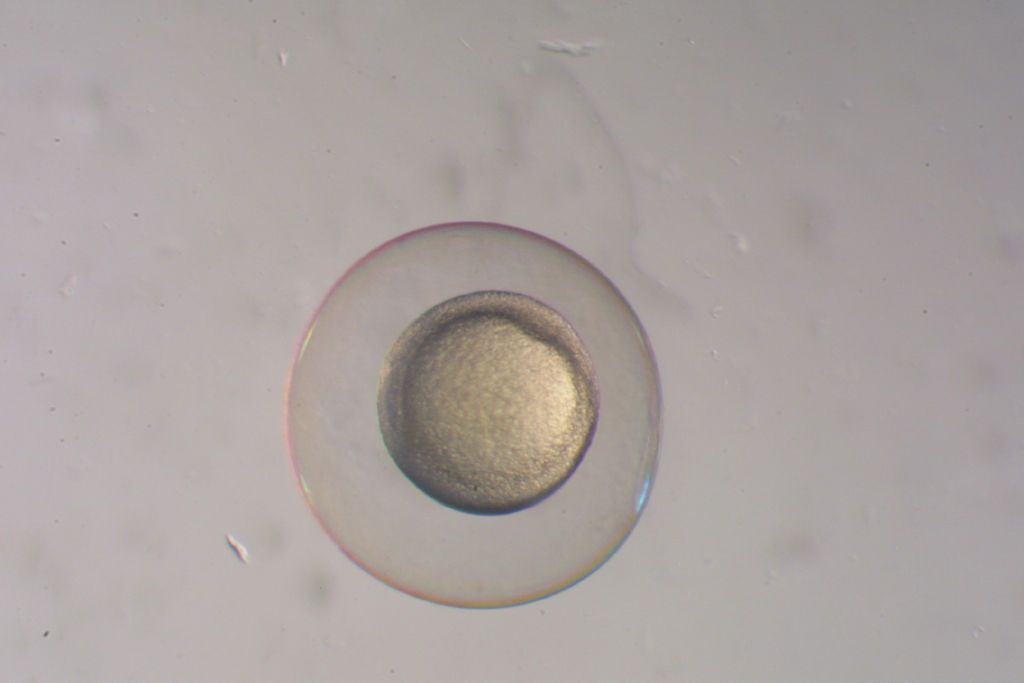
While that process could effectively put fish embryos into a state of suspended animation, heating them back up again remained a problem. As they warm, there’s an intermediary point between the ideal glasslike state and room temperature where ice crystals can begin to form again. And those crystals can damage the cellular material, leaving it incapable of further development.
“We needed to thaw them much faster,” Hagedorn said. “Using the tools we had in 2011 . . . I hit a wall.”
For a while she gave up.
And that’s how things might have remained were it not for a chance encounter at a cryopreservation conference sometime in 2013 where she heard a presentation by John Bischof, a mechanical engineering professor at the University of Minnesota.
As Bischof tells it, he’d been presenting on an unrelated topic involving iron oxide nanoparticles, which his lab has used in the safe rewarming of human tissue for transplantation. His research clicked with Hagedorn, prompting her to think about its potential for non-mammalian applications.
“She said: What can you do to help me with the embryos,” Bischof recalls.
That initial question gave birth to a complex, ongoing interdisciplinary collaboration—one in which both Hagedorn and Bischof insist on the importance of the other’s work.
Their results, published this week in the journal ACS Nano indicate that it might be possible to safely rewarm frozen fish embryos after all.
The inspiration for their work came from the efforts of a now-deceased scientist named Peter Mazur who thought it might be possible to rewarm frozen embryos with lasers. (Yes, lasers.) While the idea was potentially sound, it’s challenging, Hagedorn told me, to get lasers to convey heat to biological material. Together with another researcher named Fritz Kleinhans, however, Mazur figured out that it might be possible to introduce another substance into the solution with the embryo, one that would pick up heat from the laser and transfer it to the biological matter.
In Mazur’s case, that meant carbon black in the form of India ink, a substance that absorbs and conveys heat well—and one that, Kleinhans says, you can simply buy on Amazon.com. If it was placed around a frozen mouse embryo, for example, a single laser pulse could almost instantaneously bring the cellular material to room temperature, bypassing the intermediary phase of warming where ice crystals threaten to form. Kleinhans says that during the earlier phase of Hagedorn’s work she had hoped that the technique might work for zebrafish embryos as well. Alas, they were still too large, and by the time that exterior heat made its way to the center, fatal ice crystals were already forming.
As Hagedorn, Bischof, and their collaborators write in their new paper, however, there was another way. Spreading India ink on the outside of the embryo may not have been enough, but what if they inserted some other responsive material within before freezing? To do so, they settled on gold nanorods—minuscule molecular structures, orders of magnitude smaller than a human hair—which they microinject along with antifreeze agents into the embryo prior to preservation, employing the methods Hagedorn had worked out years before.
As the researchers write in their paper, “These nanoparticles can effectively generate heat when the laser wavelength matches the gold nanoparticle’s surface plasmon resonance energy.” That’s a complicated way of saying the nanorods could absorb and amplify the energy from a brief flash of light.
Gold, like many other substances, exhibits different properties on the nanoscale than it does in bulk. A well-calibrated millisecond laser pulse can suddenly heat up an embryo by way of the gold distributed throughout it, reheating it at the astonishing rate of 1.4 x 107 °C per minute, an almost unfathomable temperature that is manageable in the quick bursts that the researchers employ.
“In that one millisecond pulse of the laser, you’re going from liquid nitrogen to room temperature,” Bischof says. Significantly, unlike any method Hagedorn had attempted before, the results were hot enough—and widely distributed enough—to successfully reheat an entire zebrafish embryo at once.
With that barrier finally crossed, questions remained. Key among them was whether those embryos would still be viable. As the researchers report in their paper, a significant portion were, though not all. Of those that they defrosted, 31 percent made it just an hour after warming, 17 percent crossed the three-hour mark, and a mere 10 percent were still developing after the 24-hour mark.
While that may sound small, it’s far larger than the zero percent survival rate that earlier methods had yielded. Hagedorn hopes that future work will “enhance” those numbers further. And she remains positive about even the 10 percent figure. “A fish can produce millions of eggs, and if I were to successfully freeze 10 percent of those, that’s a really good number,” she says.
Of course, grappling with millions of eggs would require that they further transform the process for efficiency. At this point, much of that work falls on the shoulders of Bischof and others in his lab, where work is already underway to improve the “throughput” of the process, potentially turning it into a more industrial endeavor. “I think there’s going to be a number of enabling technologies that are going to be developed toward that in the coming years,” he told me.
If that work succeeds, Hagedorn thinks it could have other uses that go far beyond the humble zebrafish.
“Lots of aquaculture farmers want to freeze fish [reproductive material], because they only spawn once a year,” she said. “You have this boom and bust aspect to running their farms. If you could take the embryos out of the freezer in a more scheduled way, it would make food cheaper and more reliable.”
It may also have an impact on wildlife conservation. Hagedorn, who works primarily on coral today, thinks it might help us repair damaged reefs. She also suggests that it could ultimately restore depleted frog populations, and maybe save other species as well. Regardless of where the work takes us in the future, though, it stands as a testament to the potential of scientific collaboration today.
“At first it honestly didn’t feel real. It makes biological sense that we could do it, but it seemed like we would never get all the pieces together,” she told me. “If I had not sat down next to John at that meeting, we would never have done this. Without our joint efforts—the engineering and the biology—this wouldn’t have happened.”

