Snowflakes All Fall In One of 35 Different Shapes
The latest categorization of solid precipitation types inspired a cool graphic
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/7d/cf/7dcf119e-ec51-47ff-89b7-d776e541f9d2/42-16889330.jpg)
The stunning diversity of snowflakes gives rise to the idea that every single one is unique. While "no two flakes alike" might be an attractive metaphor, it isn’t entirely true. Yet that doesn’t stop us from peering at the intricate crystal structures caught on our mittens. It also doesn’t stop researchers from painstakingly cataloguing each and every type of crystal that might form.
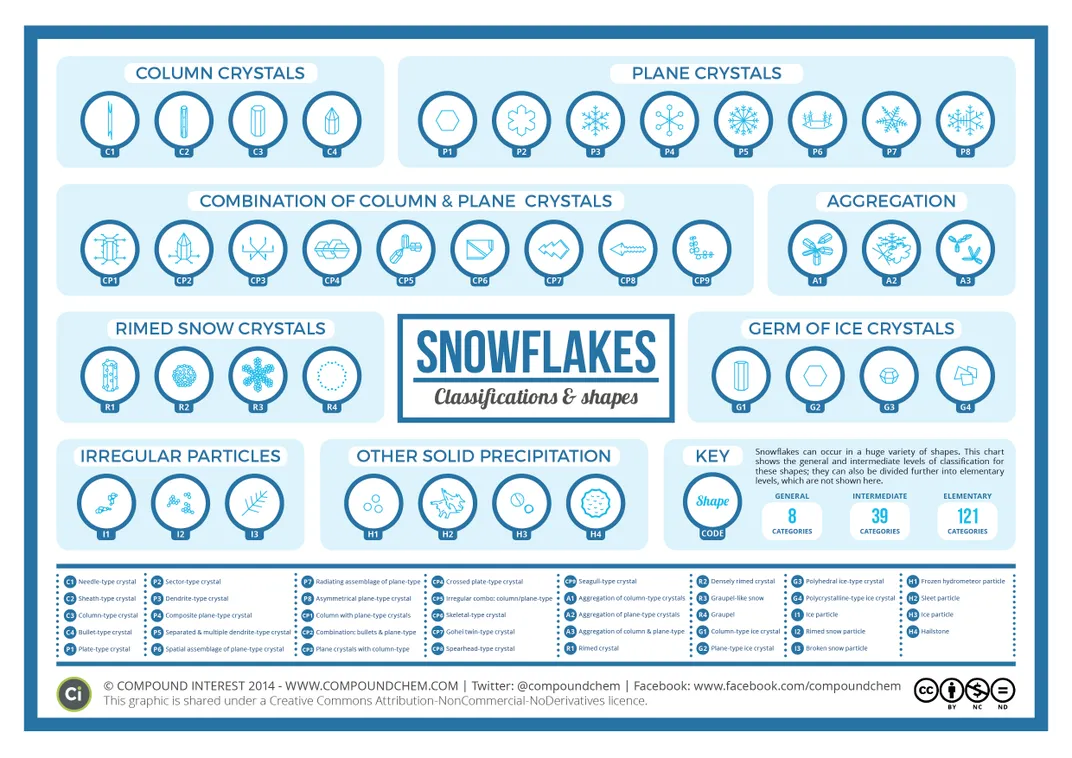
Thanks to their work, chemistry teacher Andy Brunning, who keeps the graphics and chemistry blog Compound Interest, has created a fascinating graphic that shows 39 kinds of solid precipitation, including 35 that are snow crystals or flakes. The other forms of precipitation pictured include sleet, ice, a hailstone and a frozen hydrometeor particle.
Brunning writes:
You might wonder what the shapes of snowflakes have to do with chemistry. Actually, the study of crystal structures of solids has its own discipline, crystallography, which allows us to determine the arrangement of atoms in these solids. Crystallography works by passing X-rays through the sample, which are then diffracted as they pass through by the atoms contained therein. Analysis of the diffraction pattern allows the structure of the solid to be discerned; this technique was used by Rosalind Franklin to photograph the double helix arrangement of DNA prior to Watson & Crick’s confirmation of its structure.
Previous efforts have come up with a few different numbers for the total categories of solid precipitation. The new graphic is based on work from researchers based in Japan. The 39 categories can be further broken down into 121 subtypes, Susannah Locke reports for Vox. And they all can be lumped into eight broader groups:
- Column crystals
- Plane crystals
- Combination of column & plane crystals
- Aggregation of snow crystals
- Rimed snow crystals
- Germs of ice crystals
- Irregular snow particles
- Other solid precipitation.
Kenneth Libbrecht a physicist at Caltech writes about snow crystal formation on his website:
The story begins up in a cloud, when a minute cloud droplet first freezes into a tiny particle of ice. As water vapor starts condensing on its surface, the ice particle quickly develops facets, thus becoming a small hexagonal prism. For a while it keeps this simple faceted shape as it grows.
As the crystal becomes larger, however, branches begin to sprout from the six corners of the hexagon (this is the third stage in the diagram at right). Since the atmospheric conditions (e. g. temperature and humidity) are nearly constant across the small crystal, the six budding arms all grow out at roughly the same rate.
While it grows, the crystal is blown to and fro inside the clouds, so the temperature it sees changes randomly with time.
Those temperature changes morph the arms into different shapes and give us the diverse snowflakes and crystals we see. Since all the arms endure the same fluctuations, they can grow symmetrically. In reality, most snow crystals are irregular, he writes.
Why spend all this time classifying snowflakes? As Libbrecht explains, this is really the study of how crystals form. And that knowledge can be applied to making crystals for a host of other applications—silicon and other semiconductors in computers and electronics are built of crystals, for example.
Plus, they are stunning.