The Possibilities and Risks of Genetically Altering Immune Cells to Fight Cancer
Of the ten or so patients I’ve treated with CAR-T, over half developed strange neurologic side effects ranging from headaches to seizures
:focal(1485x965:1486x966)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/df/20/df2010f3-a977-49db-a5bc-4efdd08081ec/ezgifcom-optimize.gif)
An unexpected early morning phone call from the hospital is never good news. When Joy Johnson answered, her first thought was that Sharon Birzer, her partner of 15 years, was dead. Her fears were amplified by the voice on the other end refusing to confirm or deny it. Just “come in and talk to one of the doctors,” she remembers the voice saying.
Johnson knew this was a real possibility. A few weeks earlier, she and Birzer sat in the exam room of a lymphoma specialist at Stanford University. Birzer’s cancer had grown, and fast — first during one type of chemotherapy, then through a second. Out of standard options, Birzer’s local oncologist had referred her for a novel treatment called chimeric antigen receptor T-cell therapy — or CAR-T. Birzer and Johnson knew the treatment was risky. They were warned there was a chance of death. There was also a chance of serious complications such as multi-organ failure and neurological impairment. But it was like warning a drowning person that her lifeboat could have problems. Without treatment, the chance of Birzer’s death was all but certain. She signed the consent form.
Johnson hung up the phone that early morning and sped to the hospital. She met with a doctor and two chaplains in a windowless room in the cancer ward, where happy photos of cancer “alumni” smiled down from the walls. This is getting worse and worse, Johnson thought. As she remembers it, the doctor went through the timeline of what happened for 10 minutes, explaining how Birzer became sicker and sicker, before Johnson interrupted with the thought splitting her world in two: “I need you to tell me whether she’s alive or dead.”
Birzer wasn’t dead. But she was far from okay. The ordeal began with Birzer speaking gibberish. Then came seizures so severe there was concern she wouldn’t be able to breathe on her own. When it took a few different medications to stop Birzer from seizing, her doctors sedated her, put a breathing tube down her throat, and connected her to a ventilator. Now, she was unconscious and in the intensive care unit (ICU).
Birzer was one of the early patients to receive CAR-T, a radical new therapy to treat cancer. It involved removing Birzer’s own blood, filtering for immune cells called T-cells, and genetically engineering those cells to recognize and attack her lymphoma. CAR-T made history in 2017 as the first FDA-approved gene therapy to treat any disease. After three to six months of follow-up, the trials that led to approval showed response rates of 80 percent and above in aggressive leukemias and lymphomas that had resisted chemotherapy. Patients on the brink of death were coming back to life.
This is something I often dream of seeing but rarely do. As a doctor who treats cancer, I think a lot about how to frame new treatments to my patients. I never want to give false hope. But the uncertainty inherent to my field also cautions me against closing the door on optimism prematurely. We take it as a point of pride that no field of medicine evolves as rapidly as cancer — the FDA approves dozens of new treatments a year. One of my biggest challenges is staying up to date on every development and teasing apart what should — and shouldn’t — change my practice. I am often a mediator for my patients, tempering theoretical promises with everyday realism. To accept a research finding into medical practice, I prefer slow steps showing me proof of concept, safety, and efficacy.
CAR-T, nearly three decades in the making, systemically cleared these hurdles. Not only did the product work, its approach was also unique among cancer treatments. Unlike our usual advances, this wasn’t a matter of prescribing an old drug for a new disease or remixing known medications. CAR-T isn’t even a drug. This is a one-time infusion giving a person a better version of her own immune system. When the FDA approved its use, it wasn’t a question of whether my hospital would be involved, but how we could stay ahead. We weren’t alone.
Today, two FDA-approved CAR-T products called Kymriah and Yescarta are available in more than 100 hospitals collectively across the U.S. Hundreds of clinical trials are tinkering with dosages, patient populations, and types of cancer. Some medical centers are manufacturing the cells on-site.
The FDA approved CAR-T with a drug safety program called a Risk Evaluation and Mitigation Strategy (REMS). As I cared for these patients, I quickly realized the FDA’s concerns. Of the 10 or so patients I’ve treated, more than half developed strange neurologic side effects ranging from headaches to difficulty speaking to seizures to falling unconscious. We scrambled to learn how to manage the side effects in real time.
Johnson and Birzer, who I didn’t treat personally but spoke to at length for this essay, understood this better than most. Both had worked in quality control for a blood bank and were medically savvier than the average patient. They accepted a medical system with a learning curve. They were fine with hearing “I don’t know.” Signing up for a trailblazing treatment meant going along for the ride. Twists and bumps were par for the course.
* * *
Cancer, by definition, means something has gone very wrong within — a cell has malfunctioned and multiplied. The philosophy for fighting cancer has been, for the most part, creating and bringing in treatments from outside the body. That’s how we got to the most common modern approaches: Chemotherapy (administering drugs to kill cancer), radiation (using high energy beams to kill cancer), and surgery (cutting cancer out with a scalpel and other tools). Next came the genetics revolution, with a focus on creating drugs that target a precise genetic mutation separating a cancer cell from a normal one. But cancers are genetically complex, with legions of mutations and the talent to develop new ones. It’s rare to have that one magic bullet.
Over the last decade or so, our approach shifted. Instead of fighting cancer from the outside, we are increasingly turning in. The human body is already marvelously equipped to recognize and attack invaders, from the common cold to food poisoning, even if the invaders are ones the body has never seen before. Cancer doesn’t belong either. But since cancer cells come from normal ones, they’ve developed clever camouflages to trick and evade the immune system. The 2018 Nobel Prize in Physiology or Medicine was jointly awarded to two researchers for their work in immunotherapy, a class of medications devoted to wiping out the camouflages and restoring the immune system’s upper hand. As I once watched a fellow oncologist describe it to a patient: “I’m not treating you. You are treating you.”
What if we could go one step further? What if we could genetically engineer a patient’s own immune cells to spot and fight cancer, as a sort of “best hits” of genetic therapy and immunotherapy?
Enter CAR-T. The technology uses T-cells, which are like the bouncers of the immune system. T-cells survey the body and make sure everything belongs. CAR-T involves removing a person’s T-cells from her blood and using a disarmed virus to deliver new genetic material to the cells. The new genes given to the T-cells help them make two types of proteins. The first — giving the technology its name — is a CAR, which sits on the T-cell’s surface and binds to a protein on the tumor cell’s surface, like a lock and key. The second serves as the T-cell’s caffeine jolt, rousing it to activate. Once the genetic engineering part is done, the T-cells are prodded to multiply by being placed on a rocking device that feeds them nutrients while filtering their wastes. When the cells reach a high enough number — a typical “dose” ranges from hundreds of thousands to hundreds of millions — they are formidable enough to go back into the patient. Once inside, the cancer provokes the new cells to replicate even more. After one week, a typical expansion means multiplying by about another 1,000-fold.
Practically, it looks like this: A person comes in for an appointment. She has a catheter placed in a vein, perhaps in her arm or her chest, that connects to a large, whirring machine which pulls in her blood and separates it into its components. The medical team set the T-cells aside to freeze while the rest of the blood circulates back into the patient in a closed loop. Then, the hospital ships the cells frozen to the relevant pharmaceutical company’s headquarters or transports them to a lab on-site, where thawing and manufacturing takes from a few days to a few weeks. When the cells are ready, the patient undergoes about three days of chemotherapy to kill both cancer and normal cells, making room for the millions of new cells and eradicating normal immune players that could jeopardize their existence. She then gets a day or two to rest. When the new cells are infused back into her blood, we call that Day 0.
* * *
I remember the first time I watched a patient get his Day 0 infusion. It felt anti-climactic. The entire process took about 15 minutes. The CAR-T cells are invisible to the naked eye, housed in a small plastic bag containing clear liquid.
“That’s it?” my patient asked when the nurse said it was over. The infusion part is easy. The hard part is everything that comes next.
Once the cells are in, they can’t turn off. That this may cause collateral damage was evident from the start. In 2009 — working in parallel with other researchers at Memorial Sloan Kettering Cancer Center in New York and the National Cancer Institute in Maryland — oncologists at the University of Pennsylvania opened a clinical trial for CAR-T in human leukemia patients. (Carl June, who led the CAR-T development, did not respond to Undark’s interview request.) Of the first three patients who got CAR-T infusions, two achieved complete remission — but nearly died in the process. The first was a retired corrections officer named Bill Ludwig, who developed extremely high fevers and went into multi-organ failure requiring time in the ICU. At the time, the medical teams had no idea why it was happening or how to stop it. But time passed. Ludwig got better. Then came the truly incredible part: His cancer was gone.
With only philanthropic support, the trial ran out of funding. Of the eligible patients they intended to treat, the Penn doctors only treated three. So they published the results of one patient in the New England Journal of Medicine and presented the outcomes of all three patients, including Ludwig, at a cancer conference anyway. From there, the money poured in. Based on the results, the Swiss pharmaceutical company Novartis licensed the rights of the therapy.
The next year, six-year-old Emily Whitehead was on the brink of death when she became the first child to receive CAR-T. She also became extremely ill in the ICU, and her cancer was also eventually cured. Her media savvy parents helped bring her story public, making her the poster child for CAR-T. In 2014, the FDA granted CAR-T a breakthrough therapy designation to expedite the development of extremely promising therapies. By 2017, a larger trial gave the treatment to 75 children and young adults with a type of leukemia — B-cell acute lymphoblastic leukemia — that failed to respond to chemotherapy. Eighty-one percent had no sign of cancer after three months.
In August 2017, the FDA approved a CAR-T treatment as the first gene therapy in the U.S. The decision was unanimous. The Oncologic Drugs Advisory Committee, a branch of the FDA that reviews new cancer products, voted 10 to zero in favor of Kymriah. Committee members called the responses “remarkable” and “potentially paradigm changing.” When the announcement broke, a crowd formed in the medical education center of Penn Medicine, made up of ecstatic faculty and staff. There were banners and T-shirts. “A remarkable thing happened” was the tagline, above a cartoon image of a heroic T-cell. Two months later, in October 2017, the FDA approved a second CAR-T formulation called Yescarta from Kite Pharma, a subsidiary of Gilead Sciences, to treat an aggressive blood cancer in adults called diffuse large B-cell lymphoma, the trial of which had shown a 54 percent complete response rate, meaning all signs of cancer had disappeared. In May 2018, Kymriah was approved to treat adults with non-Hodgkin lymphoma.
That year, the American Society of Clinical Oncology named CAR-T the Advance of the Year, beating out immunotherapy, which had won two years in a row. When I attended the last American Society of Hematology meeting in December 2018, CAR-T stole the show. Trying to get into CAR-T talks felt like trying to get a photo with a celebrity. Running five minutes late to one session meant facing closed doors. Others were standing room only. With every slide, it became difficult to see over a sea of smartphones snapping photos. At one session I found a seat next to the oncologist from my hospital who treated Birzer. “Look,” she nudged me. “Do you see all these ‘non-member’ badges?” I turned. Members were doctors like us who treated blood cancers. I couldn’t imagine who else would want to be here. “Who are they?” I asked. “Investors,” she said. It felt obvious the moment she said it.
For patients, the dreaded “c” word is cancer. For oncologists, it’s cure. When patients ask, I’ve noticed how we gently steer the conversation toward safer lingo. We talk about keeping the cancer in check. Cure is a dangerous word, used only when so much time has passed from her cancer diagnosis we can be reasonably certain it’s gone. But that line is arbitrary. We celebrate therapies that add weeks or months because the diseases are pugnacious, the biology diverse, and the threat of relapse looming. Oncologists are a tempered group, or so I’ve learned, finding inspiration in slow, incremental change.
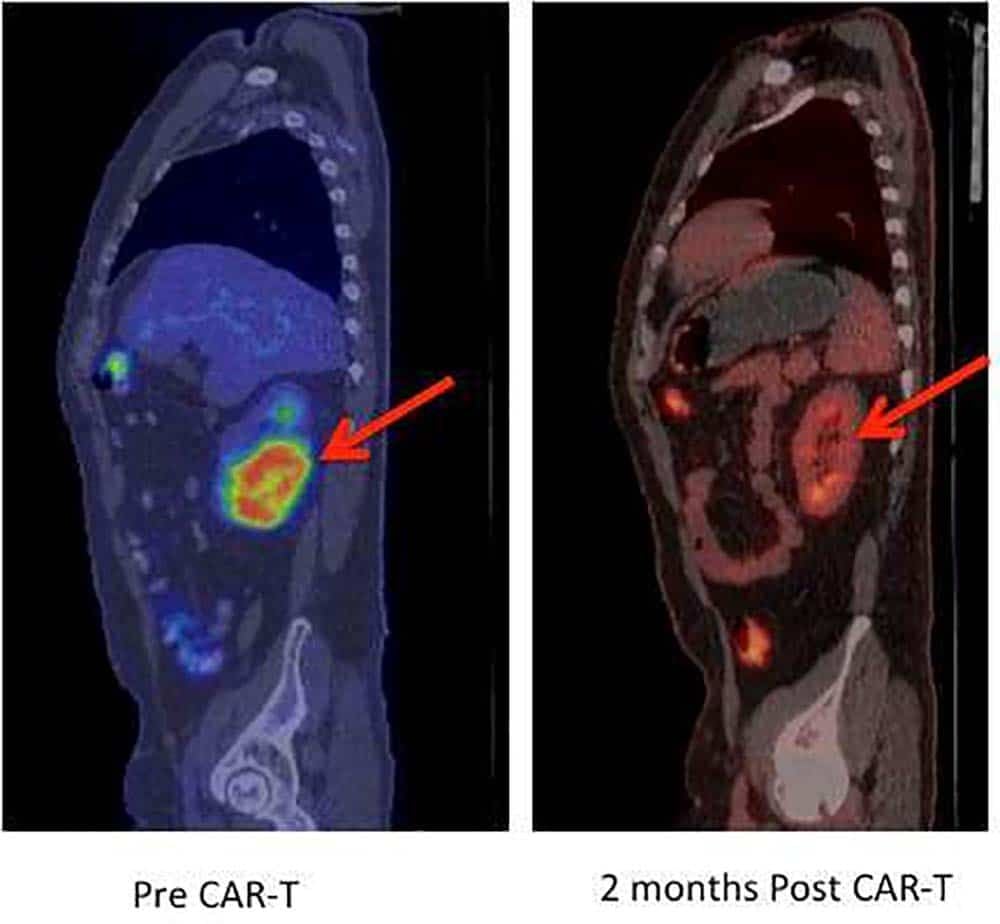
This was completely different. These were patients who would have otherwise died, and the trials were boasting that 54 to 81 percent were cancer-free upon initial follow-up. PET scans showed tumors that had speckled an entire body melt away. Bone marrow biopsies were clear, with even the most sensitive testing unable to detect disease.
The dreaded word was being tossed around — could this be the cure we’ve always wanted?
* * *
When a new drug gets FDA approval, it makes its way into clinical practice, swiftly and often with little fanfare. Under the drug safety program REMS, hospitals offering CAR-T were obligated to undergo special training to monitor and manage side effects. As hospitals worked to create CAR-T programs, oncologists like me made the all too familiar transition from first-time user to expert.
It was May 2018 when I rotated through my hospital’s unit and cared for my first patients on CAR-T. As I covered 24-hour shifts, I quickly learned that whether I would sleep that night depended on how many CAR-T patients I was covering. With each treatment, it felt like we were pouring gasoline on the fire of patients’ immune systems. Some developed high fevers and their blood pressures plummeted, mimicking a serious infection. But there was no infection to be found. When resuscitating with fluids couldn’t maintain my patients’ blood pressures, I sent them to the ICU where they required intensive support to supply blood to their critical organs.
We now have a name for this effect — cytokine release syndrome — that occurs in more than half of patients who receive CAR-T, starting with Ludwig and Whitehead. The syndrome is the collateral damage of an immune system on the highest possible alert. This was first seen with other types of immunotherapy, but CAR-T took its severity to a new level. Usually starting the week after CAR-T, cytokine release syndrome can range from simple fevers to multi-organ failure affecting the liver, kidneys, heart, and more. The activated T-cells make and recruit other immune players called cytokines to join in the fight. Cytokines then recruit more immune cells. Unlike in the early trials at Penn, we now have two medicines to dampen the effect. Steroids calm the immune system in general, while a medication called tocilizumab, used to treat autoimmune disorders such as rheumatoid arthritis, blocks cytokines specifically.
Fortuity was behind the idea of tocilizumab: When Emily Whitehead, the first child to receive CAR-T, developed cytokine release syndrome, her medical team noted that her blood contained high levels of a cytokine called interleukin 6. Carl June thought of his own daughter, who had juvenile rheumatoid arthritis and was on a new FDA-approved medication that suppressed the same cytokine. The team tried the drug, tocilizumab, in Whitehead. It worked.
Still, we were cautious in our early treatments. The symptoms of cytokine release syndrome mimic the symptoms of severe infection. If this were infection, medicines that dampen a patient’s immune system would be the opposite of what you’d want to give. There was another concern: Would these medications dampen the anti-cancer activity too? We didn’t know. Whenever a CAR-T patient spiked a fever, I struggled with the question — is it cytokine release syndrome, or is it infection? I often played it safe and covered all bases, starting antibiotics and steroids at the same time. It was counterintuitive, like pressing both heat and ice on a strain, or treating a patient simultaneously with fluids and diuretics.
The second side effect was even scarier: Patients stopped talking. Some, like Sharon Birzer, spoke gibberish or had violent seizures. Some couldn’t interact at all, unable to follow simple commands like “squeeze my fingers.” How? Why? At hospitals across the nation, perfectly cognitively intact people who had signed up to treat their cancer were unable to ask what was happening.
Our nurses learned to ask a standardized list of questions to catch the effect, which we called neurotoxicity: Where are we? Who is the president? What is 100 minus 10? When the patients scored too low on these quizzes, they called me to the bedside.

In turn, I relied heavily on a laminated booklet, made by other doctors who were using CAR-T, which we tacked to a bulletin board in our doctors’ workroom. It contained a short chart noting how to score severity and what to do next. I flipped through the brightly color-coded pages telling me when to order a head CT-scan to look for brain swelling and when to place scalp electrodes looking for seizures. Meanwhile, we formed new channels of communication. As I routinely called a handful of CAR-T specialists at my hospital in the middle of the night, national consortiums formed where specialists around the country shared their experiences. As we tweaked the instructions, we scribbled updates to the booklet in pen.
I wanted to know whether my experience was representative. I came across an abstract and conference talk that explored what happened to 277 patients who received CAR-T in the real world, so I emailed the lead author, Loretta Nastoupil, director of the Department of Lymphoma and Myeloma at the University of Texas MD Anderson Cancer Center in Houston. Fortuitously, she was planning a trip to my university to give a talk that month. We met at a café and I asked what her research found. Compared to the earlier trials, the patients were much sicker, she said. Of the 277 patients, more than 40 percent wouldn’t have been eligible for the very trials that got CAR-T approved. Was her team calling other centers for advice? “They were calling us,” she said.
Patients included in clinical trials are carefully selected. They tend not to have other major medical problems, as we want them to survive whatever rigorous new therapy we put them through. Nastoupil admits some of it is arbitrary. Many criteria in the CAR-T trials were based on criteria that had been used in chemotherapy trials. “These become standard languages that apply to all studies,” she said, listing benchmarks like a patient’s age, kidney function, and platelet count. “But we have no idea whether criteria for chemotherapy would apply to cellular therapy.”
Now, with a blanket FDA approval comes clinical judgment. Patients want a chance. Oncologists want to give their patients a chance. Young, old, prior cancer, heart disease, or liver disease — without strict trial criteria, anyone is fair game.
When I was making rounds at my hospital, I never wandered too far from these patients’ rooms, medically prepared for them to crash at any moment. At the same time, early side effects made me optimistic. A bizarre truism in cancer is that side effects may bode well. They could mean the treatment is working. Cancer is usually a waiting game, requiring months to learn an answer. Patients and doctors alike seek clues, but the only real way to know is waiting: Will the next PET scan show anything? What are the biopsy results?
CAR-T was fundamentally different from other cancer treatments in that it worked fast. Birzer’s first clue came just a few hours after her infusion. She developed pain in her lower back. She described it as feeling like she had menstrual cramps. A heavy burden of lymphoma lay in her uterus. Could the pain mean that the CAR-T cells had migrated to the right spot and started to work? Her medical team didn’t know, but the lead doctor’s instinct was that it was a good sign.
Two days later, her temperature shot up to 102. Her blood pressure dropped. The medical team diagnosed cytokine release syndrome, as though right on schedule, and gave her tocilizumab.
Every day, the nurses would ask her questions and have her write simple sentences on a slip of paper to monitor for neurotoxicity. By the fifth day, her answers changed. “She started saying things that were crazy,” Johnson explained.
One of Birzer's sentences was “guinea pigs eat greens like hay and pizza.” Birzer and Johnson owned two guinea pigs, so their diet would be something Birzer normally knew well. So Johnson tried to reason with her: “They don’t eat pizza.” And Birzer replied, “They do eat pizza, but only gluten-free.”
Johnson remembers being struck by the certainty in her partner’s delirium. Not only was Birzer confused, she was confident she was not. “She was doubling down on everything,” Johnson described. “She was absolutely sure she was right.”
Johnson vividly remembers the evening before the frightening early-morning phone call that brought her rushing back to the hospital. Birzer had said there was no point in Johnson staying overnight; she would only watch her be in pain. So Johnson went home. After she did, the doctor came by multiple times to evaluate Birzer. She was deteriorating — and fast. Her speech became more and more garbled. Soon she couldn’t name simple objects and didn’t know where she was. At 3 a.m., the doctor ordered a head CT to make sure Birzer wasn’t bleeding into her brain.
Fortunately, she wasn’t. But by 7 a.m. Birzer stopped speaking altogether. Then she seized. Birzer’s nurse was about to step out of the room when she noticed Birzer’s arms and legs shaking. Her eyes stared vacantly and she wet the bed. The nurse called a code blue, and a team of more doctors and nurses ran over. Birzer was loaded with high-dose anti-seizure medications through her IV. But she continued to seize. As nurses infused more medications into her IV, a doctor placed a breathing tube down her throat.
Birzer’s saga poses the big question: Why does CAR-T cause seizures and other neurologic problems? No one seemed to know. My search of the published scientific literature was thin, but one name kept cropping up. So I called her. Juliane Gust, a pediatric neurologist and scientist at Seattle Children’s Hospital, told me her investigations of how CAR-T affects the brain were motivated by her own experiences. When the early CAR-T trials opened at her hospital in 2014, she and her colleagues began getting calls from oncologists about brain toxicities they knew nothing about. “Where are the papers?” she remembered thinking. “There was nothing.”
Typically, the brain is protected by a collection of cells aptly named the blood-brain-barrier. But with severe CAR-T neurotoxicity, research suggests, this defense breaks down. Gust explained that spinal taps on these patients show high levels of cytokines floating in the fluid surrounding the spine and brain. Some CAR-T cells circulate in the fluid too, she said, but these numbers do not correlate with sicker patients. CAR-T cells are even seen in the spinal fluid of patients without any symptoms.
What does this mean? Gust interprets it as a patient’s symptoms having more to do with cytokines than the CAR-T cells. “Cytokine release syndrome is the number one risk factor” for developing neurotoxicity over the next few days, she said. The mainstay for neurotoxicity is starting steroids as soon as possible. “In the beginning we didn’t manage as aggressively. We were worried about impairing the function of the CAR-T,” she added. “Now we give steroids right away.”
But the steroids don’t always work. Several doses of steroids didn’t prevent Birzer from seizing. The morning after Johnson’s alarming phone call, after the meeting at the hospital when she learned what had happened, a chaplain walked her from the conference room to the ICU. The first day, Johnson sat by her partner’s bedside while Birzer remained unconscious. By the next evening, she woke up enough to breathe on her own. The doctors removed her breathing tube, and Birzer looked around. She had no idea who she was or where she was.
Birzer was like a newborn baby, confused and sometimes frightened by her surroundings. She frequently looked like she was about to say something, but she couldn’t find the words despite the nurses and Johnson’s encouragement. One day she spoke a few words. Eventually she learned her name. A few days later she recognized Johnson. Her life was coming back to her, though she was still suspicious of her reality. She accused the nurses of tricking her, for instance, when they told her Donald Trump was president.
She took cues from the adults around her on whether her actions were appropriate. The best example of this was her “I love you” phase. One day, she said it to Johnson in the hospital. A few nurses overheard it and commented on how sweet it was. Birzer was pleased with the reaction. So she turned to the nurse: “I love you!” And the person emptying the trash: “I love you!” Months later, she was having lunch with a friend who asked, “Do you remember when you told me you loved me?” Birzer said, “Well, I stand by that one.”
When she got home, she needed a walker to help with her shakiness on her feet. When recounting her everyday interactions, she would swap in the wrong people, substituting a friend for someone else. She saw bugs that didn’t exist. She couldn’t hold a spoon or a cup steady. Johnson would try to slow her down, but Birzer was adamant she could eat and drink without help. “Then peas would fly in my face,” Johnson said.
Patients who experience neurotoxicity fall into one of three categories. The majority are impaired but then return to normal without long-term damage. A devastating handful, less than 1 percent, develop severe brain swelling and die. The rest fall into a minority that have lingering problems even months out. These are usually struggles to think up the right word, trouble concentrating, and weakness, often requiring long courses of rehabilitation and extra help at home.
As Birzer told me about her months of rehab, I thought how she did seem to fall somewhere in the middle among the patients I’ve treated. On one end of the spectrum was the rancher who remained profoundly weak a year after his infusion. Before CAR-T, he walked across his ranch without issue; six months later, he needed a walker. Even with it, he fell on a near weekly basis. On the other end was the retired teacher who couldn’t speak for a week – she would look around her ICU room and move her mouth as though trying her hardest — and then woke up as though nothing happened. She left the hospital and instantly resumed her life, which included a recent trip across the country. In hindsight, I remember how we worried more about giving the therapy to the teacher than the rancher, as she seemed frailer. Outcomes like theirs leave me with a familiar humility I keep learning in new ways as a doctor: We often can’t predict how a patient will do. Our instincts can be just plain wrong.
I asked Gust if we have data to predict who will land in which group. While we can point to some risk factors — higher burdens of cancer, baseline cognitive problems before therapy — “the individual patient tells you nothing,” she confirmed.
So we wait.
* * *
Doctors like me who specialize in cancer regularly field heart-wrenching questions from patients. They have read about CAR-T in the news, and now they want to know: What about me? What about my cancer?
So, who gets CAR-T? That leads to the tougher question — who doesn’t? That depends on the type of cancer and whether their insurance can pay.
CAR-T is approved to treat certain leukemias and lymphomas that come from the blood and bone marrow. Since the initial approval, researchers have also set up new CAR-T trials for all sorts of solid tumors from lung cancer to kidney cancer to sarcoma. But progress has been slow. While some promising findings are coming from the lab and in small numbers of patients on early phase trials, nothing is yet approved in humans. The remarkable responses occurring in blood cancers just weren’t happening in solid tumors.
Cancer is one word, but it’s not one disease. “It’s easier to prove why something works when it works than show why it doesn’t work when it doesn’t work,” said Saar Gill, a hematologist and scientist at the University of Pennsylvania who co-founded a company called Carisma Therapeutics using CAR-T technology against solid tumors. That was his short answer, at least. The longer answer to why CAR-T hasn’t worked in solid cancers involves what Gill believes are two main barriers. First, it’s a trafficking problem. Leukemia cells tend to be easier targets; they bob through the bloodstream like buoys in an ocean. Solid tumors are more like trash islands. The cancer cells stick together and grow an assortment of supporting structures to hold the mound together. The first problem for CAR-T is that the T-cells may not be able to penetrate the islands. Then, even if the T-cells make it in, they’re faced with a hostile environment and will likely die before they can work.
At Carisma, Gill and his colleagues look to get around these obstacles though a different immune cell called the macrophage. T-cells are not the only players of the immune system, after all. Macrophages are gluttonous cells that recognize invaders and engulf them for destruction. But studies have shown they cluster in solid tumors in a way T-cells don’t. Gill hopes genetically engineered macrophages can be the stowaways that sneak into solid tumor and attack from the inside out.
Another big challenge, even for leukemias and lymphomas, is resistance, where the cancers learn to survive the CAR-T infusion. While many patients in the trials achieved remission after a month, we now have two years’ worth of data and the outlook isn’t as rosy. For lymphoma, that number is closer to 40 percent. Patients celebrating cures initially are relapsing later. Why?
The CAR-T cells we use target a specific protein on cancer cells. But if the cancer no longer expresses that protein, that can be a big problem, and we’re finding that’s exactly what’s happening. Through blood testing, we see that many patients who relapse lose the target.
Researchers are trying to regain the upper hand by designing CAR-Ts to target more than one receptor. It’s an old idea in a new frame: An arms race between our medicines and the illnesses that can evolve to evade them. Too much medical precision in these cases is actually not what we want, as it makes it easier for cancer to pinpoint what’s after it and develop an escape route. So, the reasoning goes, target multiple pieces at once. Confuse the cancer.
Then there’s the other dreaded “c” word: Cost. Novartis’ Kymriah runs up to $475,000 while Kite Pharma’s Yescarta is $373,000. That covers manufacturing and infusion. Not included is the minimum one-week hospital stay or any complications.
They are daunting numbers. Some limitations on health care we accept — maybe the patients are too sick; maybe they have the wrong disease. The wrong cost is not one we as a society look kindly upon. And drug companies shy away from that kind of attention.
Cost origins in medicine are notoriously murky. Novartis, confident in its technology, made an offer to offset the scrutiny in CAR-T. If the treatment didn’t work after one month, the company said it wouldn’t send a bill.
Not everyone agrees that cost is an issue. Gill, for example, believes the concern is over-hyped. It’s not “a major issue,” he told me over the phone. “Look, of course — [with] health care in this country, if you don’t have insurance, then you’re screwed. That is no different when it comes to CAR-T as it is for anything else,” he said. The cost conversation must also put CAR-T in context. Gill went on to list what these patients would be doing otherwise — months of chemotherapy, bone marrow transplants, hospital stays for cancer-associated complications and the associated loss of income as patients and caregivers miss work. These could add up to far more than a one-time CAR-T infusion. A bone marrow transplant, for example, can cost from $100,000 to more than $300,000. The cancer-fighting drug blinatumomab, also used to treat relapsed leukemia, costs $178,000 a year. “Any discussion of cost is completely irresponsible without weighing the other side of the equation,” Gill said.
How the system will get on board is another question. Logistics will be an issue, Gill conceded. The first national Medicare policy for covering CAR-T was announced in August 2019, two years after the first product was approved. The Centers for Medicare and Medicaid Services has offered to reimburse a set rate for CAR T-cell infusion, and while this figure was recently raised, it remains less than the total cost. Despite the expansion of medical uses, at some centers referrals for CAR-T are dropping as hospitals worry it’s a net loss. And while most commercial insurers are covering CAR-T therapies, companies less accustomed to handling complex therapies can postpone approval. Ironically, the patients considering CAR-T are the ones for whom the window for treatment is narrowest. A delay of even a few weeks can mean the difference between a cure and hospice.
This, of course, poses a big problem. A breakthrough technology is only as good as its access. A major selling point of CAR-T — besides the efficacy — is its ease. It’s a one-and-done treatment. Engineered T-cells are intended to live indefinitely, constantly on the alert if cancer tries to come back. Compare that to chemotherapy or immunotherapy, which is months of infusions or a pill taken indefinitely. CAR-T is more akin to surgery: Cut it out, pay the entire cost upfront, and you’re done.
Birzer was lucky in this respect. I asked her and Johnson if cost had factored into their decision to try CAR-T. They looked at each other. “It wasn’t an issue,” said Johnson. They remembered getting a statement in the mail for a large sum when they got home. But Birzer had good insurance. She didn’t pay a cent.
* * *
One year after Birzer’s infusion, I met her and Johnson at a coffee shop near their home in San Francisco. They had saved a table. Johnson had a newspaper open. Birzer already had her coffee, and I noticed her hand trembling as she brought it to her mouth. She described how she still struggles to find exactly the right words. She sometimes flings peas. But she’s mostly back to normal, living her everyday life. She has even returned to her passion, performing stand-up comedy, though she admitted that at least for general audiences: “My jokes about cancer didn’t kill.”
People handed a devastating diagnosis don’t spend most of their time dying. They are living, but with a heightened awareness for a timeline the rest of us take for granted. They sip coffee, enjoy their hobbies, and read the news while also getting their affairs in order and staying on the lookout, constantly, for the next treatment that could save them.
Hoping for a miracle while preparing to die are mutually compatible ideas. Many of my patients have become accustomed to living somewhere in that limbo. It is humbling to witness. They hold out hope for a plan A, however unlikely it may be, while also adjusting to the reality of a plan B. They live their lives; and they live in uncertainty.
I see patients in various stages of this limbo. In clinic, I met a man with multiple myeloma six months after a CAR-T trial that supposedly cured him. He came in with a big smile but then quietly began praying when it was time to view PET results. He asked how the other patients on the trial were doing, and I shared the stats. While percentages don’t say anything about an individual experience, they’re also all patients have to go on. When someone on the same treatment dies, it’s shattering for everyone. Was one person the exception, or a harbinger of another’s fate? Who is the outlier?
I look at these patients and think a sober truth: Before CAR-T, all would likely die within six months. Now, imagine taking 40 percent and curing them. Sure, a naysayer might point out, it’s only 40 percent. What’s the hype if most still succumb to their cancer? But there was nothing close to that before CAR-T. I agree with how Gill described it: “I think CAR-T cells are like chemotherapy in the 1950s. They’re not better than chemotherapy — they’re just different.” For an adversary as tough as cancer, we’ll take any tool we can get.
There remain many questions. Can we use CAR-T earlier in a cancer’s course? Lessen the side effects? Overcome resistance? Streamline manufacturing and reimbursement? Will it work in other cancers? Patients will sign up to answer.
For now, Birzer seems to be in the lucky 40 percent. Her one-year PET scan showed no cancer. I thought of our last coffee meeting, where I had asked if she ever worried she wouldn’t return to normal. She didn’t even pause. “If you’re not dead,” she said, “you’re winning.”
* * *
Ilana Yurkiewicz, M.D., is a physician at Stanford University and a medical journalist. She is a former Scientific American Blog Network columnist and AAAS Mass Media Fellow. Her writing has also appeared in Aeon Magazine, Health Affairs, and STAT News, and has been featured in "The Best American Science and Nature Writing."
This article was originally published on Undark. Read the original article.