Tiny Robots Can Clear Clogged Arteries
Engineers at Drexel University are developing micro-swimmers that loosen arterial plaque and release drugs into the bloodstream to prevent future buildup
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/57/bb/57bb9ff6-094e-4bdf-bc1e-5d7ff3307ac3/istock_000060351344_small.jpg)
Surgeons will soon be deploying armies of tiny robots to perform microsurgeries throughout the body. Though this may seem like science fiction, a research team from Drexel University has developed a micro-robotic technology that is being considered for an important mission—drilling through clogged arteries.
Atrial plaques form when fat, cholesterol, calcium and other substances are deposited on the inner walls of the arteries, which carry blood throughout the body. Over time, these arteries harden and narrow. This process called atherosclerosis limits the ability of oxygen rich blood to reach vital organs and increases risk for heart attack or stroke. Although the cause of atherosclerosis is unknown, a combination of habits (such as activity level, smoking and diet), genetic risk factors and age contribute to its development. Two conventional surgical approaches for blocked arteries are angioplasty and bypass surgery. During an angioplasty, a vascular surgeon inflates a small balloon inside the blood vessel and inserts a metal mesh tube called a stent to hold the arteries open and improve blood flow. By contrast, a bypass surgery involves the rerouting of blood flow by using unblocked veins or arteries to bypass the narrowed artery.
This new innovation in nanomedicine, however, takes the form of small microbeads that join together to form a corkscrew-like structure capable of navigating the treacherous waters of the body’s vascular system. The micro-swimmers are made up of tiny iron oxide beads as small as 200 nanometers, joined together in a chain. These beads are “composed of inorganic, biocompatible materials that will not trigger an immunological response,” says MinJun Kim, a professor in Drexel University’s College of Engineering.
To induce movement through the blood stream, the chain is exposed to a finely calibrated external magnetic field. The rotation of this field causes the chain to form a spinning helical structure that propels itself through the blood stream. The properties of this magnetic field also help control the speed, direction and size of the micro-swimmer chain (affecting the force with which it moves) based on the nature of the arterial occlusion.
“The use of micro-robots in medicine is really a brand new field, which requires a strong multidisciplinary research background,” says Kim.
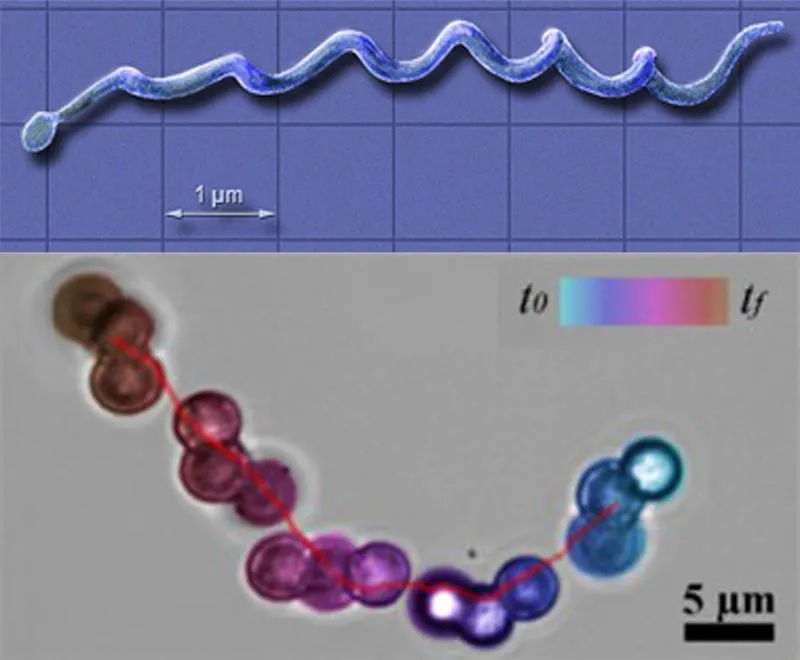
The unique design for the micro-swimmer was inspired by nature itself—a microorganism called Borrelia burgdorferi. The spiral structure of this bacterium, which is responsible for causing Lyme disease, allows it to easily infiltrate bodily fluids and cause widespread damage.
In order to remove arterial plaques, the scientists will use a catheter to deliver the micro-swimmers and a tiny vascular drill to clear the occluded artery. Upon deployment, the micro-swimmers will launch the initial attack loosening the hardened plaque, which will in turn be finished off by the surgical drill. After the surgery, the biodegradable beads are designed to release anticoagulant drugs into the bloodstream to help stymie future plaque buildup.
“Current treatments for chronic total occlusion are only about 60 percent successful,” Kim said in a press release. “We believe that the method we are developing could be as high as 80 to 90 percent successful and possibly shorten recovery time.”
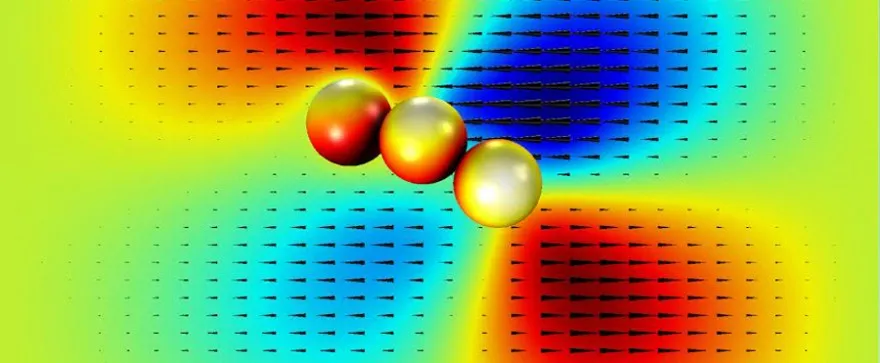
The research team had to overcome several challenges to develop functional robots at such a microscopic scale. “The microscopic world is completely different than the macroscopic world that we all live in,” says Kim. “We use inertia to move around in the macroscopic world, but on the microscopic level inertia is not useful for movement.” As a result, the scientists had to use asymmetric (or chiral) structures for the micro-swimmers. “We can create single-bead and two-bead micro-swimmers, but when we apply the magnetic field they cannot move at all because their structures are symmetric. So in order to create a non-symmetric structure we needed to use at least three beads,” says Kim.
Another obstacle the researchers faced was the complex fluid properties of the blood. Unlike water, blood is referred to as a non-Newtonian fluid, meaning its viscosity (or resistance to flow) of the fluid is not directly proportional to the speed with which it flows. As a result, the algorithms for the control of the micro-swimmers that Kim and his team developed were based on non–linear fluid dynamics and were much more elaborate. “This non-linear control makes it much more difficult to manipulate robots at the microscale,” says Kim.
The Drexel scientists have joined the Daegu Gyeongbuk Institute of Science and Technology to expand this technology for everyday use by cardiovascular surgical teams. So far, the micro-swimmers have only been tested in artificial blood vessels. The international research effort, an $18 million project funded by the Korea Evaluation Institute of Industrial Technology, has recruited top engineers from 11 other institutions in the United States, Korea and Switzerland. They hope to have the technology in human clinical trials within four years.
In addition to the use of the micro-swimmers as plumbing devices for the arteries, the researchers have been investigating other potential biomedical applications, such as more targeted drug therapies and higher resolution imaging technology. “For example, the beads could be used to penetrate directly into difficult-to-reach cancer tumor cells where the drug will be released into the target, thereby maximizing drug efficiency,” says Kim.
Kim’s interest in the field of nanotechnology was sparked by the 1966 science fiction movie Fantastic Voyage and its Steven Spielberg-directed remake Innerspace. Both of these films involve the miniaturization of a human-piloted submarine that is subsequently injected into the human body on a life-saving mission.
“I watched Innerspace when I was in high school in 1987. The film contains numerous concepts of micro-robotics and nanomedicine that have served as an inspiration to both myself and other researchers in this field,” says Kim. “I am excited to be part of a project that is involved in bringing this science fiction into reality.”

