Building a Bionic Pancreas
A device that tracks blood sugar and automatically administers insulin and glucagon could take some pressure off Type 1 diabetes patients and their parents
Today, algorithms everywhere get to know a person’s needs and customize experiences accordingly. Music services tailor playlists. Retailers offer specific product recommendations. Social media platforms are constantly calculating the next best content to display, in real time.
Boston University biomedical engineer Edward Damiano and his colleagues, including senior research scientist Firas El-Khatib, have used similar logic to tackle a medical challenge: how to automatically regulate insulin and glucagon levels in Type 1 diabetes patients, in real time.
The team is developing and testing, with a group at Massachusetts General Hospital, a device called a bionic pancreas. While the name may conjure up visions of Iron Man and super-bots, the actual product is an adaptation of common tools that many Type 1 diabetes patients already use.
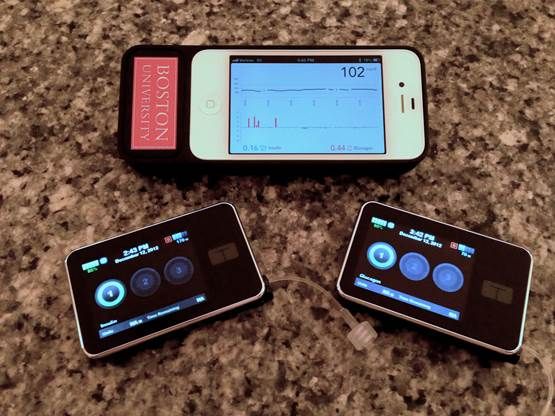
Currently, patients wear external insulin pumps, often on their abdomens. The portable pump supplies insulin to its users through a catheter or plastic tube inserted under the skin of their bellies, but it must be checked regularly to make sure it's doing so at the right rates. Together, the pump, the catheter and a steel or Teflon needle that goes under the skin make up what's called an "infusion set." Patients also rely on continuous glucose sensors. The tiny sensor is inserted under the skin along with its transmitter, much like the pump, and held in place with a Band-Aid-like adhesive. It monitors glucose rates and transmits this information to an external device using an electric signal. Right now, patients must also manually track the information the sensor provides.
The bionic pancreas uses a control algorithm to connect these two pieces. It acts as a bridge between the continuous glucose sensor and the pump, taking out the constant need to check on either of them.
How it works: the sensor captures an individual’s blood sugar and sends that data to a smartphone. The control algorithm, which runs on the smartphone, uses the data it has just received to determine the patient’s insulin and glucagon needs. The smartphone uses a Bluetooth signal to send this information to two pumps the patient is wearing, one for insulin and one for glucagon, which then administer the necessary amounts of each.
The Algorithm
The backbone of the device is the control algorithm Damiano and his team have devised. It starts by getting to know a few key parameters about patients—their age, their weight and, most importantly, the composition of their blood sugar and how it changes. Once it has this information, the algorithm makes a precise recommendation, every five minutes, 24 hours a day, for a total of 288 pivotal daily decisions, about how much insulin or glucagon their pumps should release into a patient’s bloodstream.
“We’re excited about developing an approach that can reduce the burden of diabetes,” says Steven Russell, principal investigator on the clinical team.
Diabetics need insulin injections when their blood sugar is too high and glucagon when it’s too low, to prevent conditions like hyperglycemia and hypoglycemia. "Dead in bed" syndrome is a rare but sudden, fatal fluctuation in blood sugar that can occur in while a young person with Type 1 Diabetes is sleeping. Currently, diabetes patients have to consistently and manually monitor their blood sugar to insure that it doesn’t spike or drop to dangerous levels. According to Saleh Adi, founder and director of the Madison Clinic for Pediatric Diabetes at University of California, San Francisco, the average patient checks his or her blood sugar between 4 to 10 times daily.
Daily Life With a Bionic Pancreas
As it stands today, a user must calibrate the bionic pancreas twice a day by pricking an index finger and providing a drop of blood to communicate glucose levels before breakfast and dinner. These values are used as reference points. A wearer can also announce meals, forewarning the device of upcoming changes in blood sugar. Throughout the day, the system will aim to get a patient as close as possible to his or her target glucose level. Users must replace their glucagon and insulin supplies daily by refilling the reservoirs in their pumps, although the team hopes this will become less frequent as more scientific advances are made in the field. The end goal is to develop a bionic pancreas that is capable of running completely autonomously.
“As you continue to change on a daily timescale, this thing will continue to adapt with you on a timescale that’s relevant,” says Damiano.
This system is one of the first that’s been able to administer both insulin and glucagon. Previous versions by the team along with other devices by Cambridge University, UC Santa Barbara and University of Virginia were only able to provide insulin due to how unstable glucagon is in solution.
A Personal Cause
Damiano’s 15-year-old son, David, has Type 1 diabetes. His diagnosis, as an infant, is what inspired Damiano to create this device.
“When my son was about a year old, it occurred to me that there may be a way I could play a role in improving his care,” says Damiano, who had been working on a mathematical model of blood flow in the body.
His work with El-Khatib on the bionic pancreas began in 2001, a time when the technology it required was still under development. An insulin pump already existed, but a continuous glucose sensor that could detect blood sugar levels under the skin was just emerging. Damiano focused on the piece that he knew he could change. “My lab took on the smarts of the system,” he says.
While his team has been working on this aspect of the device, there have been concurrent advances in the sensors and other elements needed to make this invention work. Companies including Dexcom and Medtronic have refined sensors that continuously track blood sugar. Yash Sabharwal and his team at Xeris Pharmaceuticals have developed a way to stabilize glucagon in solution.
“An artificial pancreas with insulin only is like trying to drive a car where you have an accelerator and no brake,” says Sabharwal, chief operating officer at Xeris Pharmaceuticals. “We’ve developed a glucagon formulation that can be stable for two years, compared to the current solution that needs to be mixed in real time.”
Testing the Device
In 2004, after he left University of Illinois for a professorship at Boston University, Damiano began testing his control algorithm in diabetic pigs. He gauged how accurately it could track their blood sugar levels and recommend the right dosages of insulin or glucagon.
After some positive results, Damiano met Russell in 2006, and together they obtained FDA clearance for their first human study. They have been conducting clinical trials ever since, including some that test the device on adults at home and children at summer camp.

The team has been able to study how the device functions and adapts to an active lifestyle by enabling trial participants to “be themselves” and experience regular routines, foods and exercises. While doing so, they’ve found the bionic pancreas to be more effective than a pump system that is manually operated.
“We’ve gone from running the algorithm on a laptop with pigs to running it on a laptop with humans to running it on an iPhone, so people can carry it around with them,” says Damiano.

Damiano and Russell will conduct trials with the University of Massachusetts, Massachusetts General Hospital, Stanford University and University of North Carolina at Chapel Hill through 2017. A study in 2016 will look at the impact of using a bionic pancreas on patients over the course of a year.
“In our clinical trials, there are all kinds of glitches that happen, because it’s a mechanical contraption,” says Damiano, citing sensors timing out, empty insulin cartridges and poor connections between the different parts. Alarms to alert the wearer when something malfunctions are in place to mitigate these issues, but the team is looking for ways to prevent them.
The Next Step: A Fully Integrated Device
Damiano is striving to have a fully integrated device—one single unit the size of an iPhone 5 with an insulin pump, glucagon pump, sensor and receiver in a battery-powered infusion set—ready in time for his son’s departure for college in 2018.
“Type 1 diabetes asks a unique amount of people. I can’t think of any other disease where we hand the drug to the patient and say, ‘You decide how much to take,’” says Russell. “We have the opportunity to change the paradigm of diabetes care.”
“People today manage their blood sugar in the dark,” says Damiano.
Helping those with Type 1 diabetes is Damiano’s first priority, but hopefully, he says, his team’s work will come to benefit Type 2 diabetes patients and, later on, improve the accuracy of insulin drips that are used in hospital settings.
When a fully-functioning bionic pancreas is available, Type 1 diabetes patients and parents of children with the condition won’t have to think about blood sugar every second.
“If a five-year-old runs 100 yards, you might have to adjust his insulin,” says Adi. “If we can take all of this away, we can restore spontaneity.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/profile.jpg)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/profile.jpg)