A Smart Sensor Could Detect Glaucoma Before Your Doctor Does
A pair of Washington researchers could be first to implant an electronic sensor—designed to give real-time analysis of the disease—directly into the eye
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/0a/ba/0aba7033-1b0f-4209-95ee-ad2ad29ef92a/42-21209349.jpg)
Glaucoma, a group of diseases that damage the optic nerve, affects more than two million Americans and is the world's second leading cause of blindness.
Those at risk for developing the disease—usually anyone over the age of 60 or those with a family history—typically go to their doctors for screenings three or four times a year. Tueng Shen, a professor of ophthalmology at the University of Washington, says that isn't good enough.
“[Current methods] limit our ability to screen people, and sometimes we don’t discover changes until long after they’ve occurred,” she says.
Her solution, which she developed with fellow professor Karl Böhringer: a prototype implant that screens for early warning signs in real time, so that doctors can begin treatment more proactively than ever before.
Outside of photo-diode-based artificial retinas, this is the first time anyone has attempted to implant an electronic sensor directly into an eye. The closest researchers and companies have come is embedding intelligence into contact lenses. One system developed at the University of Michigan gives the wearer night vision, and one from Google uses sensors to monitor glucose levels.
The primary cause of glaucoma is an increase in pressure inside the eye caused by a buildup of fluid. The added pressure can do irreparable damage to the optic nerve and prevent it from functioning.
During a traditional glaucoma screening, a doctor numbs the patient’s eye and applies a small puff of air. The force pushes on the cornea, which indicates the level of pressure within the eye.
“It’s very similar to how you see how well a basketball [is inflated],” Shen explains. “You squeeze it.”
But, Shen points out, the amount of pressure can change quickly, which means patients should have more-regular monitoring.
“It’s like testing blood sugars,”Shen says. “It’s a progressive process. It’s the rise and fall and rise and fall and general unsteadiness that will cause damage.”
Normally, it would take a lot for a patient to detect a problem on his or her own. Pressure inconsistency would need to be extreme and prolonged before a patient might have any noticeable symptoms, including extreme pain and vomiting.
An implant would allow doctors to monitor the issue and start treatment before it's too late, Shen says.
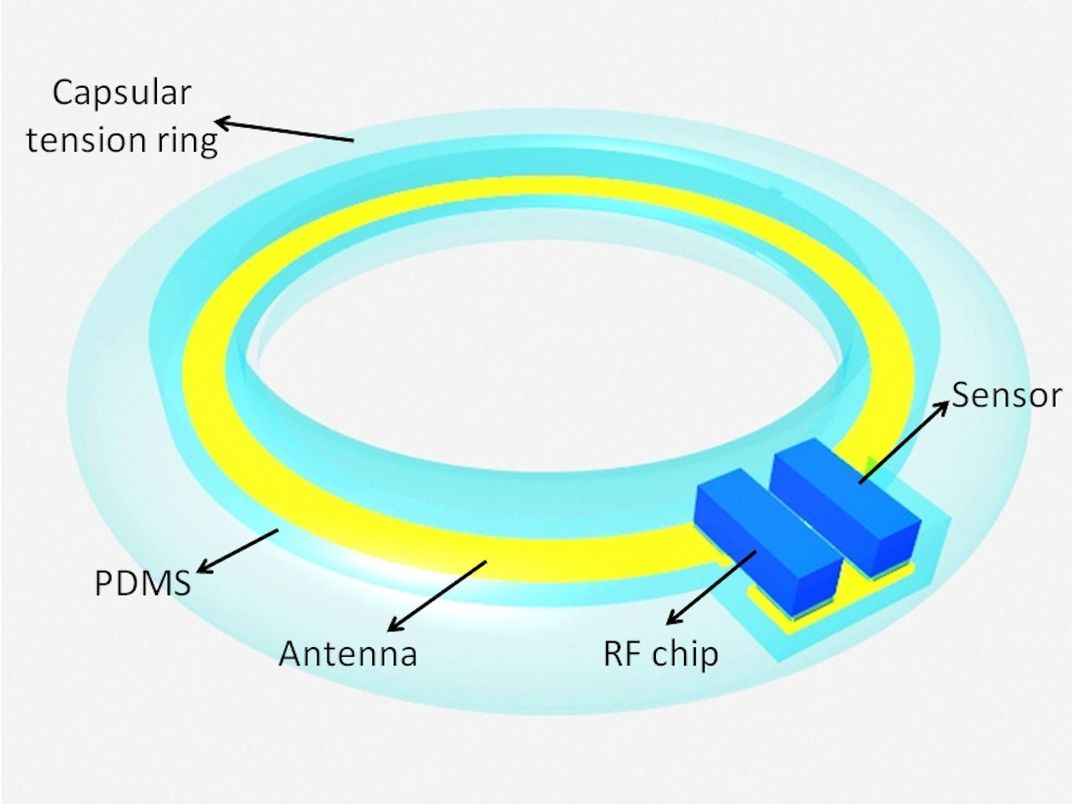
Böhringer, who developed the device with Shen, says the design of the implant is very simple: It consists of a pressure sensor, a small processor and an antenna. Researchers intend for it to be embedded within an artificial lens during cataract surgery, after which the antenna, wrapped around the perimeter of the lens, transmits data from the sensor to an outside device.
The same antenna also gathers energy to wirelessly power the chip—similar to the way an electric toothbrush juices up.
Böhringer envisions an exterior control device, perhaps the size of a cellphone, will provide power and gather and transmit pressure data.
“Maybe a future cellphone itself could have the capability,” he posits, “but that’s something we’d have to look into.”
Because the system is designed to embed in existing cataract implants, patients will not have to undergo extra surgery. It’s a good starting point, Shen says, since risk factors for both diseases are similar. Doctors perform about three million cataract surgeries every year, a number that research shows will increase steadily over the coming decades.
Both professors are quick to stress that their implant is still an early-stage prototype, or a proof of concept.
“This is not ready for implanting right now,” Böhringer says, “It just has all the components to show that it is doable.”
Their prototype is also much larger than an in-vivo implant would need to be; it’s measured in centimeters, and it will need to scale down to millimeters to fit inside the eye.
It could be as long as five years before they’re ready for human testing, Böhringer says. But applications for Shen and Böhringer’s system could eventually stretch beyond Glaucoma. The sensor can already detect changes in temperature, so they may be able to modify it to track things like the eye’s acidity level, among other measures of health.
“This is more of a platform,” Shen says, “we’re building a foundation—a whole group of different ways to approach health."
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/me.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/me.jpg)