Your Monthly Menstrual Cycle, Reenacted on a Microchip
Bodies are complicated, but they’re no match for persistent bioengineers
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/d4/b0/d4b03859-c83b-46ad-a421-2555fbe13e92/istock-587776890.jpg)
The human body is really complicated—and the situation becomes even more complex when it comes to the female reproductive cycle. So how best to study this intricate system? A group of researchers think they have the answer: Recreate it in a dish.
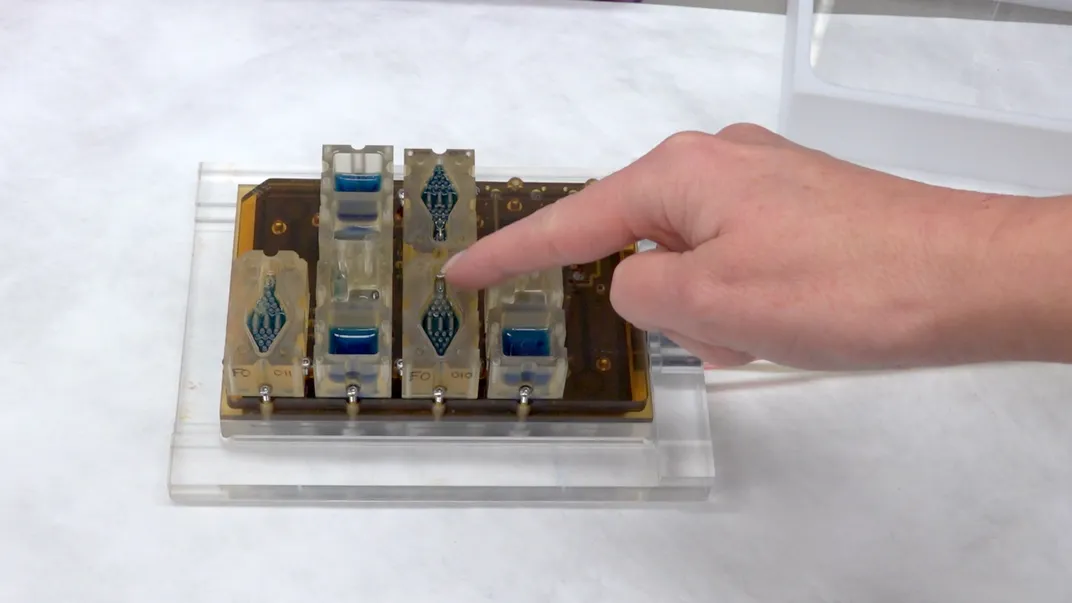
To accomplish this feat, researchers created a platform that put all of the tissues involved in the female reproductive cycle onto microchips. Each chip contained different tissues—ovary tissues from mice and human fallopian tube tisues, uterine lining (endometrium), liver and cervix tissue. Then they figured out how to make these tissues interact over a month-long period. The team recently published their results in the journal Nature Communications.
The menstrual cycle on a chip system solves a problem scientists have long had while trying to study drug interactions in the human body—and how those drugs could affect reproduction. With current technology, it is both impossible and unethical to study the full reproductive cycle in real time. Growing tissues from the reproductive tract in the lab is difficult, too. And when people die, it’s no longer possible to study the delicate interactions between the reproductive tract tissues along with the effects of hormones.
Animal studies present another problem—scientists can study their reproduction, but medicines affect animals differently than they do humans. Consider the case of thalidomide, says Nathaniel Huebsch, senior scientist at the University of California Berkeley’s Healy Laboratory, which specializes in bioengineering and “organ-on-a-chip” technology. He tells Smithsonian.com that the drug is an example of a time when scientists got it wrong. It was tested on animals and didn't appear to cause any negative effects on rodents or their offspring. But once it made it to market in the 1960s, researchers discovered that the drug caused devastating birth defects in humans.
“The same exact cue can do dramatically different things depending on the context of the cells receiving that cue,” he says.
Studying the female reproductive cycle may be hard, but the new system could one day make it much easier. The researchers used microfluidics—technology that manipulates fluids across tiny channels, offering opportunities to both control and better study their properties. In this case, electromagnetically pumped fluids allowed the different tissues to communicate with one another in the same way a reproductive tract would.
The tiny system worked. When the researchers introduced hormones into the mix, the tissues responded like they would inside a body—and the interactions could be sustained over the course of an entire 28-day-long “menstrual cycle.” Inside the tiny chips, different reproductive tissues did their thing, secreting hormones at different levels over the course of the month and even contributing to ovarian follicle growth.
Huebsch, who was not involved in the research, says that the new system shows promise. “I can see a path toward scaling this up,” he tells Smithsonian.com. “If you could do this at a large enough scale, you could really do some discovery.” And the researchers agree. In the paper, they write that the tool could improve both the pace and quality of reproductive, toxicology and drug research.
Their work, however, is far from done. The new system only mimicked hormones and not other factors like immunity or the support of offspring, so it’s a far cry from replicating all of the complicated factors the reproductive system handles every day. The human body remains almost unimaginably complicated—but bit by bit, scientists are moving toward better ways to mimic and study it.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/erin.png)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/erin.png)