The Origins of Life
A mineralogist believes he’s discovered how life’s early building blocks connected four billion years ago
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/origins-of-life-Bob-Hazen-631.jpg)
A hilly green campus in Washington, D.C. houses two departments of the Carnegie Institution for Science: the Geophysical Laboratory and the quaintly named Department of Terrestrial Magnetism. When the institution was founded, in 1902, measuring the earth’s magnetic field was a pressing scientific need for makers of nautical maps. Now, the people who work here—people like Bob Hazen—have more fundamental concerns. Hazen and his colleagues are using the institution’s “pressure bombs”—breadbox-size metal cylinders that squeeze and heat minerals to the insanely high temperatures and pressures found inside the earth—to decipher nothing less than the origins of life.
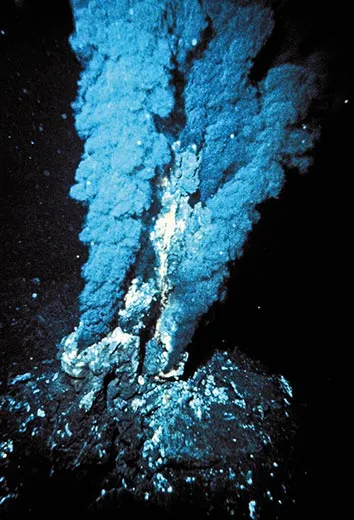
Hazen, a mineralogist, is investigating how the first organic chemicals—the kind found in living things—formed and then found each other nearly four billion years ago. He began this research in 1996, about two decades after scientists discovered hydrothermal vents—cracks in the deep ocean floor where water is heated to hundreds of degrees Fahrenheit by molten rock. The vents fuel strange underwater ecosystems inhabited by giant worms, blind shrimp and sulfur-eating bacteria. Hazen and his colleagues believed the complex, high-pressure vent environment—with rich mineral deposits and fissures spewing hot water into cold—might be where life began.
Hazen realized he could use the pressure bomb to test this theory. The device (technically known as an “internally heated, gas media pressure vessel”) is like a super-high-powered kitchen pressure cooker, producing temperatures exceeding 1,800 degrees and pressures up to 10,000 times that of the atmosphere at sea level. (If something were to go wrong, the ensuing explosion could take out a good part of the lab building; the operator runs the pressure bomb from behind an armored barrier.)
In his first experiment with the device, Hazen encased a few milligrams of water, an organic chemical called pyruvate and a powder that produces carbon dioxide all in a tiny capsule made of gold (which does not react with the chemicals inside) that he had welded himself. He put three capsules into the pressure bomb at 480 degrees and 2,000 atmospheres. And then he went to lunch. When he took the capsules out two hours later, the contents had turned into tens of thousands of different compounds. In later experiments, he combined nitrogen, ammonia and other molecules plausibly present on the early earth. In these experiments, Hazen and his colleagues created all sorts of organic molecules, including amino acids and sugars—the stuff of life.
Hazen’s experiments marked a turning point. Before them, origins-of-life research had been guided by a scenario scripted in 1871 by Charles Darwin himself: “But if (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, etc., present, that a proteine compound was chemically formed ready to undergo still more complex changes....”
In 1952, Stanley Miller, a graduate student in chemistry at the University of Chicago, attempted to create Darwin’s dream. Miller set up a container holding water (representing the early ocean) connected by glass tubes to one containing ammonia, methane and hydrogen—a mixture scientists of the day thought approximated the early atmosphere. A flame heated the water, sending vapor upward. In the atmosphere flask, electric sparks simulated lightning. The experiment was such a long shot that Miller’s adviser, Harold Urey, thought it a waste of time. But over the next few days, the water turned deep red. Miller had created a broth of amino acids.
Forty-four years later, Bob Hazen’s pressure bomb experiments would show that not just lightning storms but also hydrothermal vents potentially could have sparked life. His work soon led him to a more surprising conclusion: the basic molecules of life, it turns out, are able to form in all sorts of places: near hydrothermal vents, volcanoes, even on meteorites. Cracking open space rocks, astrobiologists have discovered amino acids, compounds similar to sugars and fatty acids, and nucleobases found in RNA and DNA. So it’s even possible that some of the first building blocks of life on earth came from outer space.
Hazen’s findings came at an auspicious time. “A few years before, we would have been laughed out of the origins-of-life community,” he says. But NASA, then starting up its astrobiology program, was looking for evidence that life could have evolved in odd environments—such as on other planets or their moons. “NASA [wanted] justification for going to Europa, to Titan, to Ganymede, to Callisto, to Mars,” says Hazen. If life does exist there, it’s likely to be under the surface, in warm, high-pressure environments.
Back on earth, Hazen says that by 2000 he had concluded that “making the basic building blocks of life is easy.” A harder question: How did the right building blocks get incorporated? Amino acids come in multiple forms, but only some are used by living things to form proteins. How did they find each other?
In a windowed corner of a lab building at the Carnegie Institution, Hazen is drawing molecules on a notepad and sketching the earliest steps on the road to life. “We’ve got a prebiotic ocean and down in the ocean floor, you’ve got rocks,” he says. “And basically there’s molecules here that are floating around in solution, but it’s a very dilute soup.” For a newly formed amino acid in the early ocean, it must have been a lonely life indeed. The familiar phrase “primordial soup” sounds rich and thick, but it was no beef stew. It was probably just a few molecules here and there in a vast ocean. “So the chances of a molecule over here bumping into this one, and then actually a chemical reaction going on to form some kind of larger structure, is just infinitesimally small,” Hazen continues. He thinks that rocks—whether the ore deposits that pile up around hydrothermal vents or those that line a tide pool on the surface—may have been the matchmakers that helped lonely amino acids find each other.
Rocks have texture, whether shiny and smooth or craggy and rough. Molecules on the surface of minerals have texture, too. Hydrogen atoms wander on and off a mineral’s surface, while electrons react with various molecules in the vicinity. An amino acid that drifts near a mineral could be attracted to its surface. Bits of amino acids might form a bond; form enough bonds and you’ve got a protein.
Back at the Carnegie lab, Hazen’s colleagues are looking into the first step in that courtship: Kateryna Klochko is preparing an experiment that—when combined with other experiments and a lot of math—should show how certain molecules stick to minerals. Do they adhere tightly to the mineral, or does a molecule attach in just one place, leaving the rest of it mobile and thereby increasing the chances it will link up to other molecules?
Klochko gets out a rack, plastic tubes and the liquids she needs. “It’s going to be very boring and tedious,” she warns. She puts a tiny dab of a powdered mineral in a four-inch plastic tube, then adds arginine, an amino acid, and a liquid to adjust the acidity. Then, while a gas bubbles through the solution, she waits...for eight minutes. The work may seem tedious indeed, but it takes concentration. “That’s the thing, each step is critical,” she says. “Each of them, if you make a mistake, the data will look weird, but you won’t know where you made a mistake.” She mixes the ingredients seven times, in seven tubes. As she works, “The Scientist” comes on the radio: “Nooooobody saaaaid it was easyyyy,” sings Coldplay vocalist Chris Martin.
After two hours, the samples go into a rotator, a kind of fast Ferris wheel for test tubes, to mix all night. In the morning, Klochko will measure how much arginine remains in the liquid; the rest of the amino acid will have stuck to the mineral powder’s tiny surfaces.
She and other researchers will repeat the same experiment with different minerals and different molecules, over and over in various combinations. The goal is for Hazen and his colleagues to be able to predict more complex interactions, like those that may have taken place in the earth’s early oceans.
How long will it take to go from studying how molecules interact with minerals to understanding how life began? No one knows. For one thing, scientists have never settled on a definition of life. Everyone has a general idea of what it is and that self-replication and passing information from generation to generation are key. Gerald Joyce, of the Scripps Research Institute in La Jolla, California, jokes that the definition should be “something like ‘that which is squishy.’”
Hazen’s work has implications beyond the origins of life. “Amino-acids-sticking-to-crystals is everywhere in the environment,” he says. Amino acids in your body stick to titanium joints; films of bacteria grow inside pipes; everywhere proteins and minerals meet, amino acids are interacting with crystals. “It’s every rock, it’s every soil, it’s the walls of the building, it’s microbes that interact with your teeth and bones, it’s everywhere,” Hazen says.
At his weekend retreat overlooking the Chesapeake Bay, Hazen, 61, peers through binoculars at some black-and-white ducks bobbing around in circles and stirring the otherwise still water. He thinks they’re herding fish—a behavior he’s never seen before. He calls for his wife, Margee, to come take a look: “There’s this really interesting phenomenon going on with the buffleheads!”
Living room shelves hold things the couple has found nearby: beach glass, a basketful of minerals, and fossilized barnacles, coral and great white shark teeth. A 15-million-year-old whale jawbone, discovered on the beach at low tide, is spread out in pieces on the dining room table, where Hazen is cleaning it. “It was part of a living, breathing whale when this was a tropical paradise,” he says.
Hazen traces his interest in prehistory to his Cleveland childhood, growing up not far from a fossil quarry. “I collected my first trilobite when I was 9 or 10,” he says. “I just thought they were cool,” he says of the marine arthropods that went extinct millions of years ago. After his family moved to New Jersey, his eighth-grade science teacher encouraged him to check out the minerals in nearby towns. “He gave me maps and he gave me directions and he gave me specimens, and my parents would take me to these places,” says Hazen. “So I just got hooked.”
After taking a paleontology class together at the Massachusetts Institute of Technology, Hazen and Margee Hindle, his future wife, started collecting trilobites. They now have thousands. “Some of them are incredibly cute,” says Hazen. “This bulbous nose—you want to hug them.”
There are trilobites all over Hazen’s office and a basement guest room at the Hazens’ Bethesda, Maryland, home—they cover shelves and fill desk drawers and cabinets. There’s even trilobite art by his now grown children, Ben, 34, who is studying to be an art therapist, and Liz, 32, a teacher. “This is the ultimate cute trilobite,” he says, reaching into a cabinet and taking out a Paralejurus. “How can you not love that?”
Hazen calls himself a “natural collector.” After he and Margee bought a picture frame that just happened to hold a photograph of a brass band, they started buying other pictures of brass bands; eventually they wrote a history of brass bands—Music Men—and a time in America when almost every town had its own. (Bob has played trumpet professionally since 1966.) He has also published a collection of 18th-and 19th-century poems about geology, most of which, he says, are pretty bad (“And O ye rocks! schist, gneiss, whate’er ye be/Ye varied strata, names too hard for me”). But the couple tend not to hold on to things. “As weird as this sounds, as a collector, I’ve never been acquisitive,” Bob says. “To have been able to hold them and study them up close is really a privilege. But they shouldn’t be in private hands.” Which is why the Hazen Collection of Band Photographs and Ephemera, ca. 1818-1931, is now at the National Museum of American History. Harvard has the mineral collection he started in eighth grade, and the Hazens are in the process of donating their trilobites to the National Museum of Natural History.
After considering, for some time, how minerals may have helped life evolve, Hazen is now investigating the other side of the equation: how life spurred the development of minerals. He explains that there were only about a dozen different minerals—including diamonds and graphite—in dust grains that pre-date the solar system. Another 50 or so formed as the sun ignited. On earth, volcanoes emitted basalt, and plate tectonics made ores of copper, lead and zinc. “The minerals become players in this sort of epic story of exploding stars and planetary formation and the triggering of plate tectonics,” he says. “And then life plays a key role.” By introducing oxygen into the atmosphere, photosynthesis made possible new kinds of minerals—turquoise, azurite and malachite, for example. Mosses and algae climbed onto land, breaking down rock and making clay, which made bigger plants possible, which made deeper soil, and so on. Today there are about 4,400 known minerals—more than two-thirds of which came into being only because of the way life changed the planet. Some of them were created exclusively by living organisms.
Everywhere he looks, Hazen says, he sees the same fascinating process: increasing complexity. “You see the same phenomena over and over, in languages and in material culture—in life itself. Stuff gets more complicated.” It’s the complexity of the hydrothermal vent environment—gushing hot water mixing with cold water near rocks, and ore deposits providing hard surfaces where newly formed amino acids could congregate—that makes it such a good candidate as a cradle of life. “Organic chemists have long used test tubes,” he says, “but the origin of life uses rocks, it uses water, it uses atmosphere. Once life gets a foothold, the fact that the environment is so variable is what drives evolution.” Minerals evolve, life arises and diversifies, and along come trilobites, whales, primates and, before you know it, brass bands.
Helen Fields has written about snakehead fish and the discovery of soft tissue in dinosaur fossils for Smithsonian. Amanda Lucidon is based in Washington, D.C.