Can Nanotechnology Save Lives?
Harvard professor and scientific genius George Whitesides believes that nanotechnology will change medicine as we know it
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/polymer-fronds-and-spheres-631.jpg)
Finding George Whitesides is often tricky even for George Whitesides. So he keeps an envelope in his jacket pocket. “I don’t actually know where I am in general until I look at it,” he says, “and then I find that I’m in Terre Haute, and then the question really is, ‘What’s next?’” During a recent stretch, the envelope revealed that he was in Boston, Abu Dhabi, Mumbai, Delhi, Basel, Geneva, Boston, Copenhagen, Boston, Seattle, Boston, Los Angeles and Boston.
The reason Boston shows up so frequently, although not as often as his wife prefers, is that Whitesides is a professor of chemistry at Harvard University, and Boston Logan is his home airport. The reason for all the other cities is that Whitesides’ contributions to science range into biology, engineering, physiology, materials science, physics and, especially these days, nanotechnology. Other scientists, government leaders, inventors and investors worldwide want to hear from him.
Whitesides’ inventions and ideas have spawned more than a dozen companies, including drug giant Genzyme. No Harvard lab comes close to matching the number of patents attached to his name—“approximately 90,” he says. The citation “GM Whitesides” shows up more frequently in academic papers than that of nearly any other chemist in history.
So Whitesides is something like the Bono of science, though taller, more wiry and at age 70, less hirsute. A Scottish fisherman’s cap almost always covers his head, even in front of an audience. He has a deep voice, with little hint of his native Kentucky. Lately that voice has been introducing audiences to a new nanotechnology project aimed at saving lives in the developing world. “What is the cheapest possible stuff that you could make a diagnostic system out of?” he asks. “Paper.”
On a piece of paper no thicker or wider than a postage stamp, Whitesides has built a medical laboratory.
One day this past winter, Whitesides woke up in his own bed. By 9 a.m. he was in his office just off Harvard Yard. He wore his typical outfit: a pinstripe suit, white shirt, no tie. He set his fisherman’s cap on a conference table in front of a bookshelf that held The Cell, Microelectronic Materials, Physical Chemistry, Advanced Organic Chemistry and Bartlett’s Familiar Quotations.
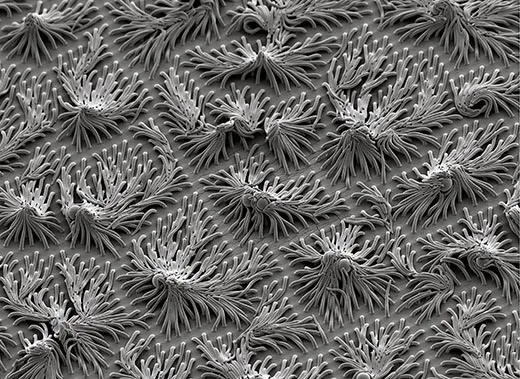
A text not on the shelf was No Small Matter: Science on the Nanoscale, a newly published coffee-table book by Whitesides and the science photographer Felice C. Frankel. It’s about truly exotic things that appear to be very large but are exceptionally, absurdly, astoundingly small—nanotubes, quantum dots, self-assembling machines.
Nanotechnology is, simply defined, the science of structures measuring between 1 nanometer, or billionth of a meter, and 100 nanometers. (The prefix “nano” comes from the Greek word for dwarf.) Still, to most people, that definition isn’t so simple. Trying to understand nanometers can rapidly induce crossed eyes. The sheet of paper these words are printed on is 100,000 nanometers thick—the diameter of a human hair, roughly the smallest object a person can see with unaided eyes. A bacterium sitting atop this paper is about 1,000 nanometers in diameter—microscopic. To see something only one nanometer in size was impossible until 1981, when two IBM physicists invented the first scanning tunneling microscope. Conventional microscopes use lenses to magnify whatever is in the line of sight. But scanning tunneling microscopes work more like a person reading Braille, moving across the surface of structures by using a tiny stylus. The physicists, who won a Nobel Prize a mere five years later, built a stylus with a tip that was just one atom across (less than one nanometer). As it moves, the stylus detects the material’s structure by recording electrical feedback, and then the microscope translates the recordings into images.
Now that really small things—right down to individual atoms—could finally be seen, Whitesides and other chemists got very interested in nanoscale materials. And what they learned amazed them. Materials this small, it turns out, have unexpected properties—we were just clueless until we could see them up close. Molecules with different surfaces—surfaces that don’t usually combine well, if at all—can suddenly bind. Glass, normally an insulator of electric currents, can conduct electricity. Materials that could not carry electric charges suddenly become semiconductors. The metal gold, in small enough particles, can appear red or blue.
“One of the fascinations of small things is that they turn out to be so alien, in spite of superficial similarities in shape or function to larger, more familiar relatives,” Whitesides writes in his book. “Discovering these differences at the smallest scale is wonderfully engrossing, and using them can change (and has changed) the world.”
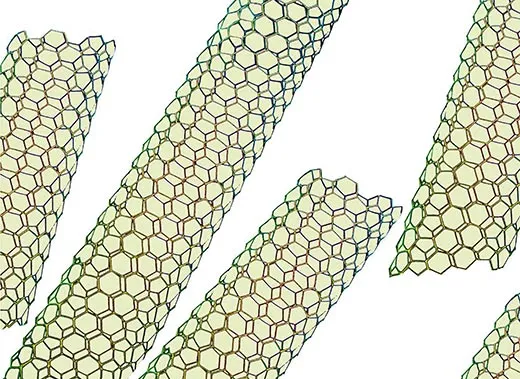
Scientists have created carbon nanotubes, hollow cylinders two nanometers or less in diameter, that turn out to be the strongest material in the world, 100 times stronger than steel with one-sixth the weight. They’ve created nanoparticles—less than 100 nanometers wide and useful for very precise biomedical images. Scientists have also made nanowires—silicon threads 10 to 100 nanometers wide and capable of converting heat to electricity. Electronics manufacturers say nanowires could make use of waste heat from computers, car engines and power plants.
Already, more than 1,000 consumer products use some form of nanotechnology (even though a 2008 report from the National Academy of Sciences urged better monitoring of potential health and environmental risks from nanotechnology). The products include stronger and lighter bike frames, fabric treatments that deflect liquids, sunscreens that repel sunlight better, memory cards for computers, and fog-resistant coatings for eyeglass lenses.
Scientists are developing nanoparticles that can deliver just the right amount of medicine to kill a tumor but nothing else around it. Other nanoparticles can detect mercury contamination in water; one day the particles may be used in filters to remove the toxic metal.
The big, life-changing stuff made from little stuff is still ahead of us. Things like batteries that can last months and power electric cars, made from nanowires built by viruses—Angela Belcher at MIT is working on that, and President Obama is so excited by the technology that he has met with her. (See “Invisible Engineers”.) A Hewlett-Packard lab, led by nanotech visionary Stan Williams, just announced a partnership with Shell to develop ultrasensitive devices to detect oil; in principle, they can register nanoscale shifts in the earth caused by movements in oil fields. Williams calls the product a “central nervous system for the earth.”
The prospect of the world fundamentally changing because of nanotechnology is still more dreamy than real, but to experts the possibilities seem almost endless. Scientists have created nanostructures that can self-assemble, meaning they can form into bigger objects with little or no outside direction. Someday these minute objects could, theoretically, build themselves into a machine that makes more nanoparticles. Already, IBM uses self-assembly techniques to produce insulation in computer chips. A center at MIT called the Institute for Soldier Nanotechnologies is working on indestructible battle armor that can react to chemical weapons.
“Everywhere you look,” Whitesides says, “you see pieces, and they’re all pointing in different directions.”
Whitesides doesn’t know exactly how he got here. Here being Harvard, this lab, this life. Growing up in a small Kentucky town, the son of a homemaker and a chemical engineer, he stuck out at school. One day, a teacher called his parents and said he’d like to talk with them about their son. Their hearts sank. “‘What’s the little bastard done now?’” Whitesides recalls of his parents’ reaction.
The teacher said, “You’ve got to get your kid out of here. I’ve arranged for him to go to Andover.”
“I had never heard of Andover,” Whitesides says now of the elite Massachusetts prep school. “I didn’t even know what it was. I didn’t know where New England was.”
And then, somehow, he ended up attending Harvard. “I don’t even remember having applied here. I just got a letter at some point admitting me. So I suppose I came here by accident.”
He went on to do graduate work at the California Institute of Technology. In the acknowledgements section of his doctoral dissertation he thanked his adviser, John D. Roberts, for “his patient direction and indirection.” Most graduate students value a mentor’s direction, Whitesides says. “In my case, he didn’t direct me at all. I don’t think I saw him in the years I was there, but we had a nice relationship.”
Whitesides taught at MIT for nearly 20 years before arriving in 1982 at Harvard, where he is something of a rarity. He is a practicing capitalist, for starters. That focuses him on real-world applications, something not all of his colleagues admire, according to Mara Prentiss, a Harvard physics professor who teaches a nanotechnology course with him. “George is greatly admired by many people, but not everyone appreciates his style,” she says. Whitesides doesn’t seem to care. “I presume it’s out there,” he says of any animosity. But he has very little time for those who think that appearing on CNN or starting companies is gauche. He says they can “just take a knitting needle and put it here”—he points at his nose—“and shove it.”
Tom Tritton, president of the Chemical Heritage Foundation, a history and educational organization in Philadelphia, says if you ask anyone in the field to list the world’s top three chemists, Whitesides will make every list. “The sheer breadth of his intellect is astounding,” Tritton says. After receiving the foundation’s highest award, the Othmer Gold Medal, Whitesides spent the day with high-school students in the city. Tritton says one student later offered this observation: “He may be a scientist, but he’s really cool.”
At the heart of almost everything Whitesides does is a contradiction: he works in complex fields of physics, chemistry, biology and engineering, using complex tools—not many people have ever wielded an atomic force microscope—and yet he is obsessed with simplicity. Ask him for an example of simplicity, and he will say, “Google.” He doesn’t mean you should Google the word “simplicity.” He means the Google home page, the spare rectangle on the white field into which millions of people type words to find information on the Internet. Whitesides is mesmerized by this box.
“But how does that work?” he says. He pauses, drawing a breath. He leans forward in his chair. His eyes get big. His forehead goes up, and with it his very large glasses. This is George Whitesides getting excited.
“You start with binary, and binary is the simplest form of arithmetic,” he says of the system of ones and zeros used to program computers. Then he launches into an impromptu historical guided tour of switches, transistors and integrated circuits before returning, finally, to Google, “which takes an idea of such incredible complexity—to organize all of humanity’s information—and puts it in this little thing, in a box.”
The idea behind Google—boiling down vast stores of knowledge into an elegant little package—is also the idea behind the thing Whitesides is now holding in his hand, a so-called lab on a chip no bigger than a postage stamp, which is designed to diagnose a variety of ailments with nearly the precision of a modern clinical laboratory.
It’s intended for health workers in remote parts of developing nations. They will place a drop of a patient’s blood or urine on the stamp; if the ailment is one of the 16 or so that the stamp can recognize, it will change color according to the affliction. Then the health worker, or even the patient, can take a picture of the stamp with a cellphone. The picture can be sent to a doctor or a lab; someday a computer program might allow the cellphone itself to make a tentative diagnosis.
“To treat disease you have to first know what you’re treating—that’s diagnostics—and then you have to do something,” Whitesides says in a standard speech he gives about the technology. “So the program that we’re involved in is something which we call diagnostics for all, or zero-cost diagnostics. How do you provide medically relevant information at as close as possible to zero cost? How do you do it?”
You start with paper, he says. It’s inexpensive. It’s absorbent. It colors easily. To turn paper into a diagnostic tool, Whitesides runs it through a wax printer. The printer melts wax onto the paper to create channels with nanometer-size molecules at the ends. These molecules react with substances in bodily fluids. The fluid “distributes itself into these various wells, or holes, and turns colors,” Whitesides explains. Think pregnancy test. A stamp that turns blue in one corner, for instance, might reveal one diagnosis; a pattern of other colors would diagnose another. The cost to produce diagnostic stamps is 10 cents each, and Whitesides hopes to make them even more cheaply. Just about any advanced cellphone with a camera could be programmed to process an image of the stamp.
“Whitesides is doing this brilliant work literally using paper,” Bill Gates said two years ago. “And, you know, it’s so cheap and it’s so simple, it could actually get out and help patients in this deep way.” Cheap and simple: Whitesides’ plan exactly. He formed a nonprofit group, Diagnostics for All, to bring the technology to developing countries. The Bill & Melinda Gates Foundation is investing in the technology to measure liver function, a test needed to ensure powerful AIDS and tuberculosis drugs don’t damage one of the body’s most important organs. Right now, testing liver function in isolated parts of the world is generally too expensive or too logistically difficult, or both. Whitesides’ stamp is also being developed to pinpoint the cause of fevers of unknown origin and identify infections. A prototype of the liver function stamp is being tested in the lab, and the early results, Whitesides says, are more than promising. The chip will begin to undergo field testing later this year.
Strolling across a stage in Boston—a rare home speaking event—Whitesides, in his fisherman’s cap, lays out his vision for how the invention will be used, sometimes in lawless places: “My view of the health care worker of the future is not a doctor, but an 18-year-old, otherwise unemployed, who has two things. He has a backpack full of these tests, and a lancet to occasionally take a blood sample, and an AK-47. And these are the things that get him through his day.”
It is a simple solution for a complicated situation, in a place far from Harvard, but working on the lab stamp is exactly where Whitesides wants to be. “What I want to do is solve problems,” he says, back at his lab, holding his lab on a chip. “And if nano is the right way of solving the problem, I’ll use that. If something else is the right way, I’ll use that. I’m not a zealot for nanotechnology. I’m not actually a zealot for anything.” Except, that is, for bringing meaning to things nobody can even see. His work could push the incredibly small architecture of nanotechnology into the architecture of everyday life.
Michael Rosenwald wrote about the search for new influenza viruses for the January 2006 issue of Smithsonian.