The Future of Antivenom May Involve Mini Lab-Grown Snake Glands
The antiquated technique used to produce antivenom requires injecting venom into horses and this new method may someday remove that step from the process
:focal(1777x259:1778x260)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/7b/3f/7b3fae23-7aba-4276-bde6-b0c1c1bd7c5a/2020_jan29_capecoralsnake.jpg)
For the first time, scientists have grown miniature, venom-producing glands in the lab using coral snake embryos, according to a news study published in the journal Cell. Why might researchers want to create artificial venom glands, you ask?
The project was initially aimed to establish proof-of-concept more than anything else. Three graduate students at the Hubrecht Institute in the Netherlands had wondered: If lab-grown organs could be made that acted like mouse and human tissues, would it work for other animals, like reptiles?
Luckily, they were working in molecular geneticist Hans Clevers’ lab. Clevers is a prominent expert in stem cell research who pioneered research on the lab-grown organ imitations—called organoids—a decade ago. Since then, researchers have created miniature human kidneys, livers, and brains in petri dishes.
On Fridays, members of the Clevers Lab are allowed to work on unstructured projects. To put their question to the test, Clevers’ students Yorick Post, Jens Puschhof, and Joep Beumer, would need a source of reptilian stem cells. As it happened, one of the researchers knew a guy: a snake breeder who could supply them with fertilized eggs, as STAT News’ Andrew Joseph reports.
They started with the egg of a Cape coral snake, removing the embryo’s venom glands and placing them in a dish. Then, they followed nearly the same protocol as they did with human cells, giving the cells ample supply of growth-inducing chemicals and storing them at a comfortable temperature—about 89 degrees Fahrenheit, about ten degrees lower than the temperature used for human cells.
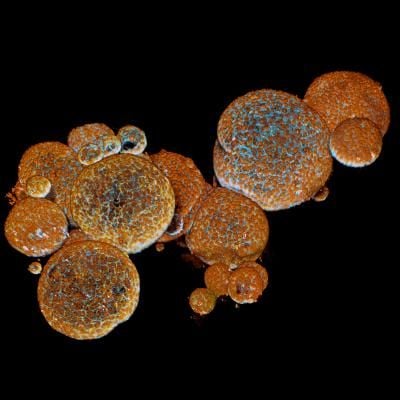
Soon, the plates held one-millimeter-long white blobs producing dangerous venom. With the organoids alive and well, the researchers told Clevers what they’d done, Leslie Nemo at Discover reports. If they’d told him beforehand, he would have told them it probably wouldn’t work, Clevers tells the Atlantic’s Ed Yong. The chemicals they used were designed for human stem cells, and very little was known about stem cells in snakes. Still, the researchers were able to grow organoids from nine species of snakes.
“It’s a breakthrough,” University of Costa Rica snake venom toxicologist José María Gutiérrez, who was not involved in the study, tells Erin Malsbury at Science magazine. “This work opens the possibilities for studying the cellular biology of venom-secreting cells at a very fine level, which has not been possible in the past,” Malsbury says.
By looking closely at the organoids, Clevers’ team gained new insight into how multiple kinds of cells work together to produce the specific mixture of toxins and proteins that results in fully-developed venom.
Venomous snake bites kill between 81,000 and 138,000 people every year, according to the World Health Organization, and cause three times as many amputations and disabilities. The antidote to a snakebite is an antivenom, but each of thousands of venomous snakes have a different bite—each requiring a unique treatment. Even snakes of the same species can produce a slightly different venoms if they live in different regions.
Right now, antivenoms are produced using much the same process as was invented in the 19th century: a live snake is “milked” for its venom, that venom is injected into a horse. Horses have been used for antivenom production for years because of their docile nature and big veins, as Douglas Main wrote for Popular Mechanics in 2016. They are first injected with adjuvant, which stimulates their immune system to produce enough antibodies to neutralize the venom. Then, researchers take a sample of their blood and separate the antivenom from other component of blood, like plasma, in a centrifuge.
Clevers’ now hopes to create a bank of dozens—and eventually thousands—of organoids from dangerous snakes and other reptiles that could aid in the effort to manufacture effective antivenoms.
"We could just sample one tissue once, and we have a source of [that snake’s] venom for eternity," Clevers tells Discover.
Clevers is working with the Dutch biologist Freek Vonk, who he calls the “Dutch Steve Irwin,” to get samples of the snake species he hopes to include in the venom gland biobank. (Vonk works at Naturalis Biodiversity Center in Leiden and also has some excellent Dutch science tunes available on Spotify.)
With venom from organoids more easily available, the hope is to skip the horse in the antitoxin-production process. Researchers could instead use the organoid-produced venom to test an array of molecules for neutralizing abilities.
“It will be interesting to see how the cost of producing venom using this system compares to the cost of purchasing venom milked from live snakes, since cost of antivenom is a key impediment to its wider use in countries where snakebite is a huge issue, like India and Nigeria,” as Bangor University molecular zoologist Anita Malhotra tells the Atlantic.
Antivenoms made from lab-grown venom glands are likely years away, but the organoids could also be a big step for studying toxin production in more detail than previously possible. With the cells isolated from the rest of the snake, researchers might be able to look at how they can produce toxic chemicals without damaging themselves, for example.
Clevers tells Discover, “We do the most interesting work when we don’t have a proposal and just try things.”