A Single Protein Is the Root of Dengue’s Virulence
But researchers who found the culprit say it could be a clue in developing a vaccine for the mosquito-borne virus
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/fd/3a/fd3ad14a-fb59-43c2-ad3c-3ec51753dd58/42-59560219.jpg)
Dengue, a mosquito-borne virus, infects some 50 million people every year and kills 22,000. Outbreaks in India and Taiwan this year have resulted in thousands of infections and a few dozen deaths. There's no treatment for dengue, and no vaccine that is completely effective.
Two teams of scientists, one at Australia’s University of Queensland and the other at University of California, Berkeley, think they have found the secret of dengue's virulence: a single protein, called nonstructural protein 1, or NS1, that acts like the poisons released by bacterial infections. The studies are in this week’s issue of Science Translational Medicine.
Dengue's symptoms include fever, rash, muscle pain and damage to the blood vessels, which causes them to leak plasma. In severe cases, the fluid loss can be deadly, and the disease in its most serious form can become dengue hemorrhagic fever, which causes nausea, vomiting and bleeding or bruising under the skin.
Most people simply recover, and then they have immunity to one of the four strains of the virus. But it wasn't clear how the hemorrhagic form of the disease was causing the damage that killed patients. "Mostly the issue is that you get leakage out of your capillaries and circulatory system," says Eva Harris, a professor of infectious diseases and virology who led the University of California, Berkeley team. "If the fluids aren't replenished, you go into decompensated shock."
Paul Young, head professor of the School of Chemistry and Biosciences at the University of Queensland, and his team found the mechanism by which NS1 operates, while Harris' was able to isolate the protein itself and use it to vaccinate mice.
Dengue wasn't always such a problem; a century ago it was limited to a very few places in the tropics. World War II changed that, because the mosquito that carries it, Aedes aegypti, was carried all over the world on cargo ships. Where once dengue needed certain animals to spread, now it relies on humans. Young noted that humans are effectively a vector for dengue in mosquitoes. Aedes aegypti likes to breed in small bodies of still water—kiddie pools, trash can lids and even the floor of a bathroom. The females bite a lot, too.
Scientists already hypothesized that severe cases were caused by an over-active immune response. Dengue, like all viruses, reproduces by taking over the machinery of host cells. In dengue's case it's cells, called dendritic cells, that alert the body to infection. The infection stimulates the cells to produce cytokines, small proteins that are part of the inflammatory response. This isn't usually deadly.
A second infection, though, with another strain of dengue, will misdirect the immune system. Antibodies from the first infection attach to the new strain of dengue, because it looks just like the first. But the new strain is slightly different, so the antibodies can't neutralize the virus completely. Instead they allow the virus to attach to the T cells that would usually kill it, and that spreads the virus further, increasing the viral load on the patient.
The result is more cytokine production. One of the functions of cytokines is to make blood vessel walls more permeable, and over-production makes them leaky. This is why second infections often lead to severe forms of the disease and bleeding. Tiny spots of blood appear on a patient’s skin and larger pockets of blood accumulate under the skin.
The mystery was which particular protein was involved and how it acted. That's where Young's team came in. Back in the early 2000s, the team had developed a way to test for dengue by measuring concentrations of the NS1 protein in the bloodstream.
"What we did find in a study in Thailand was that, if patients had high levels of NS1, they were more likely to go on to severe disease. We thought it was just a viral infection marker," Young says. "But then we asked if it was having a direct effect itself."
They decided to look more closely at NS1. That's when they found that it binds to another molecule, called toll-like receptor 4 (TLR4). That allows it to link to the cells in blood vessel walls, called endothelial cells. NS1 also stimulated immune cells to release cytokines—the over-active inflammatory response. NS1 was acting very much like a bacterial toxin.
Young says the NS1 findings mean that to treat dengue, it might be possible to use existing drugs. Some version of those that treat sepsis, for example, might work.
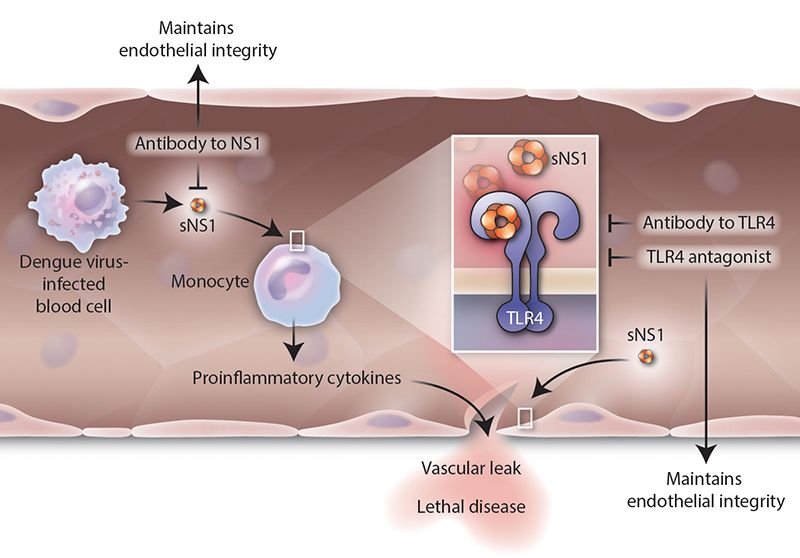
The next question was whether one could stop it. Harris' team looked at NS1's role in dengue infection more directly. They experimented on mice, infecting them with dengue, and then with the protein by itself. They used NS1 from all four strains of dengue.
The researchers found that in both cases the mice developed antibodies. They also discovered that NS1 all by itself can cause the blood vessels to leak fluid. "We thought that maybe the protein had a role in vascular leakage," Harris says.
Mice given a small amount of NS1, who showed an immune response, seemed to be protected from the virus. The reason, Harris says, is that the antibodies link to the protein itself, rather than a specific viral strain, and the NS1 produced by all four dengue strains is the same.
The protection against the virus wasn't 100 percent across the different strains of dengue, (called DENV1, DENV2, DENV3 and DENV4). In their study, Harris' team found that when inoculated with the NS1 from DENV2, protection was 100 percent from that strain. It was 75 percent from DENV1, and 60 percent from DENV3 and DENV4.
They then tested the protein and the virus on human pulmonary endothelial cells in culture. They saw that NS1 wasn't able to damage the cells when the TLR4 protein was blocked—more evidence that the NS1 that causes vascular leakage in humans.
Harris notes that their work, coupled with the findings of Young's team that TLR4 links dengue to other cells, offers important insights. "If we can target TLR4, we have a new way of making a therapy," she says, in addition to a vaccine.
There is still a lot of work to be done, she says. While they know that NS1 is the culprit, it isn't clear yet which specific piece of NS1 is the one that generates the right antibodies and damages cells. She notes that West Nile Virus also has NS1 and behaves differently. "NS1 has a whole bunch of roles that are not well understood," she says.
That said, the new work could add another weapon for public health officials to control the disease; the usual methods are focused on controlling the mosquito.
Young's and Harris' groups aren't the only ones working on dengue. Sanofi Pasteur, a company that develops, manufactures and supplies vaccines, has a new vaccine that is registered in a number of countries; approval for use could come in the next several months, says Susan Watkins, senior director of communications. The Sanofi vaccine uses an attenuated virus (it's actually a yellow fever virus with a dengue "coat").
According to a New England Journal of Medicine study, the Sanofi vaccine candidate protected on average 66 percent of volunteers aged 9 years and older against all four dengue strains, and of those, 93 percent were protected from the severe form of the disease. The Sanofi vaccine, though, doesn't offer the same level of protection against all four strains—against one type it was only 42 percent effective, while it was 77 percent effective against another.
One other advantage of using NS1 as a base for a vaccine is that it doesn't involve using the virus at all. "The FDA would be happier if we could knock out pieces of the protein that cause disease and leave those that give protection," Harris says.