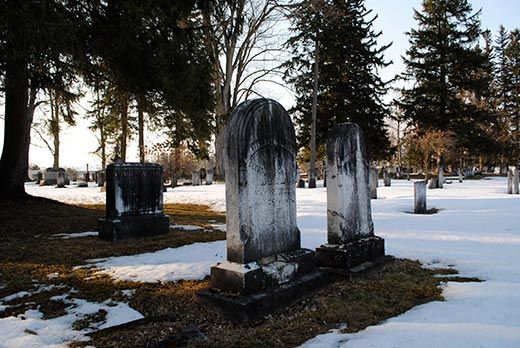Acid Rain and Our Ecosystem
More than 150 years after acid rain was first identified, scientists now see success in recovery from its damaging effects
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/acid-rain-black-crust-gravestones-Madison-Street-Cemetery-Hamilton-New-York-631.jpg)
Geologist Rich April climbs the small hill behind Colgate University and makes his way into the cemetery. He stops before a white marble pillar erected in 1852. The inscription is nearly illegible. Over time, any stone exposed to the elements will weather, April explains, but this marble has weathered unnaturally fast. The culprit? Acid rain.
April pulls a vial of acid from his pocket to demonstrate. He unscrews the cap and lets a few drops leak onto the stone, where they fizz and bubble. The rain that fell throughout the Northeast in the latter half of the 20th century wasn’t as acidic as the liquid in April’s vial, but the principle is the same. Acid eats marble. Given enough time, it can erase even words meant to last an eternity.
The effects of acid rain extend far beyond graveyards. Acid rain destroyed fish populations in lakes and streams, harmed fragile soils and damaged millions of acres of forest worldwide.
These far-reaching effects illustrate the profound impact air pollution can have on the land. But the story of acid rain is also a tale of how understanding air pollution can lead to solutions. Due to overwhelming scientific evidence linking power plant emissions to acid rain and acid rain to the death of lakes, new regulations have dramatically cut emissions and cleaned up the rain that falls on the United States.
The term ‘acid rain’ was coined in the mid-1800s, when Robert Angus Smith, a Scottish chemist working in London, noticed that rain tended to be more acidic in areas with more air pollution and that buildings crumble faster in areas where coal is burned. But it took another century for scientists to realize that acid rain was a widespread environmental problem. Scandinavian scientists began to document acidic damage to lakes and streams in the 1950s. In 1963, Gene Likens, then at Dartmouth, and colleagues began collecting and testing the pH of rainwater in New Hampshire’s White Mountains as part of an ecosystem study. They were surprised to find that it was quite acidic, but they didn’t have much basis for comparison; at that time, scientists weren’t regularly measuring the pH of rainwater.
Likens took a job at Cornell a few years later and set up instruments to collect rainwater in the Finger Lakes region and soon observed that the rain in New York was roughly as acidic as rain in New Hampshire. “That was the first clue that we had that this might be some kind of a regional phenomenon,” he says. But neither Likens nor his colleagues had a clear idea what the cause might be.
Likens won a fellowship that took him to Sweden in 1969, a serendipitous event, he says, because he met Svante Odén, a scientist at Uppsala University who had observed the same trends in Sweden that Likens had been observing in the Northeastern United States. Odén had his finger on a potential cause. “He was trying to build a case that [acid rain] might be due to emissions coming from the more industrialized areas of Europe,” Likens recalls.
Likens and his colleagues traced the emissions from coal-fired power plants and examined satellite and aircraft data, and they found a similar long-distance link. “Sure enough, the emissions were coming primarily from Midwestern states like Indiana, Ohio, Illinois and Kentucky,” Likens recalls. “They were making their way literally thousands of kilometers to New England and southeastern Canada and coming back down as acids.”
He reported his findings in Science in 1974, and the story was immediately picked up by newspapers. The phone didn’t stop ringing for months, Likens recalls. “It was that media exposure that really put acid rain on the map in North America.”
Acid rain occurs, Likens and Odén and other scientists realized, when sulfur dioxide and nitrogen oxide enter the atmosphere and react with water to form sulfuric and nitric acids. Natural sources of these gases exist—volcanoes, for instance, belch out sulfur dioxide—but the vast majority comes from the burning of fossil fuels, especially by coal-fired power plants. The tall smokestacks allow pollution to travel long distances. According to studies conducted by Likens and his colleagues, normal rainwater has a pH of 5.2. During the 1970s and 1980s, when acid rain was at its worst, scientists recorded pH levels as low as 2.1, roughly 1,000 times more acidic.
Acid rain affected many parts of the United States, but the Northeast suffered the most ecological damage. The Adirondack Mountains proved especially susceptible. Many soils contain calcium carbonate or other minerals that can neutralize acid rain before it seeps into lakes and streams. “Unfortunately the Adirondacks have almost none,” April says. As a result, lakes and streams quickly became acidic, killing fish and other aquatic animals.
In the late 1970s, researchers surveyed 217 lakes above 2,000 feet in the Adirondacks and found that 51 percent were highly acidic. The news was so grim that scientists began attempting to breed more acid-tolerant strains of trout. One New York State employee compared the area to Death Valley. A decade later, a larger study that included 849 lakes higher than 1,000 feet found that 55 percent were either completely devoid of life or on the brink of collapse.
As the scientific evidence linking acid rain to power plant emissions and ecological damage mounted, battles erupted among industry, scientists and environmentalists. “The 1980s is a period I call the ‘acid rain wars,’” Likens says. “There was huge rancorous nasty controversy.” Environmentalists from Greenpeace climbed power plant smokestacks and hung banners in protest; scientists testified before Congress about the link between emissions and acid rain, the severity of the effects, and whether proposed legislation would have an impact; and the power industry questioned the science and argued that regulations would drive electricity rates sky high.
Congress passed several amendments to the Clean Air Act in 1990 that cut emissions of sulfur dioxide through a cap-and-trade scheme. The goal was a 50 percent reduction in sulfur dioxide emissions from 1980 levels. That goal was achieved in 2008, two years before the deadline, which was set for 2010. Sulfur dioxide emissions fell from 17.3 million tons in 1980 to 7.6 million tons in 2008, less than the 8.95 million tons required by 2010.
The effect has been remarkable. Doug Burns, a scientist at the U.S. Geological Survey in Troy, New York, who directs the National Acid Precipitation Assessment Program, says the rain falling in the Northeast today is about half as acidic as it was in the early 1980s. Consequently, surface waters have become less acidic and fragile ecosystems are beginning to recover.
In many places, however, recovery has been painfully slow. Scientists now know that acid rain not only acidified lakes and streams, it also leached calcium from forest soils. That calcium depletion has had devastating effects on trees, especially sugar maples and red spruce. Acid rain leaches calcium from the needles of red spruce, making them more susceptible to cold. It also leaches calcium and magnesium from the soil, which can stress sugar maples. In addition, acid rain allows aluminum to accumulate in the soil. When trees take up aluminum, their roots can become brittle.
Some researchers have tried adding calcium back into the forests to speed recovery. April is currently involved in one such experiment in the Adirondacks. Over the past four and a half years, the calcium has penetrated only the top 15 centimeters of forest soil. “It takes a really long time for [the calcium] to get back down into the soil,” April says, so it won’t be a quick fix.
April would like to see sulfur dioxide and other emissions curtailed even further. “We still have acid rain coming in,” he says. “Some lakes look like they might be ready to come back, and if we cut the emissions more they would.”
Princeton University’s Michael Oppenheimer, who was a key player in the acid wars as chief scientist for the conservation group Environmental Defense Fund, agrees. “I think sulfur dioxide and nitrogen oxide need to be effectively eliminated,” he says. “We ought to head towards zero and see how close we can get.”
Although some effects of acid rain are lingering, most scientists consider it an environmental success story. “Science identified the problem. Science provided the guidelines for how to try to resolve the problem,” Likens says. “The success is that we have taken action as a society to try to deal with the problem.”