How to Stop a Lethal Virus
With tens of millions of lives at stake, medical researchers are racing to create a revolutionary flu vaccine before the next devastating epidemic
:focal(4282x5615:4283x5616)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/cb/b1/cbb12ae3-6e1f-4fb0-bb91-9fb886e016d7/nov2017_k02_vaccine.jpg)
In the last week of March in 2009, two children in Southern California came down with the flu. They were 9 and 10 years old, a girl and a boy, and though it was very late in flu season, they both had textbook symptoms: sudden fever, a cough and hit-by-a-truck lassitude. The kids had no connection to each other—their families lived in adjacent counties in the bottom of the state—but by chance, both of the clinics their parents took them to were participating in influenza-tracking projects run by the Centers for Disease Control and Prevention, the U.S. federal agency that monitors disease threats at home and around the world.
That was a fortunate accident, because it meant that both kids got their throats swabbed, to check which of the several strains of flu that circulate each year was making them sick. But what seemed like a routine first step quickly became a source of alarm. The two children, living more than 100 miles apart, presented with strains that were very similar to each other—but it was a new type of flu, and based on genetic evidence, it had originated in pigs. A flu strain that leaps from an animal species to infect humans is a signal for trouble; a virus the human immune system has never experienced is more likely to cause severe disease and death.
Less than two weeks after the test results came in, the United States declared a national public health emergency. The strain spread rapidly around the world, and panic followed. In June, as cases mounted worldwide, the World Health Organization declared that an influenza pandemic—the first of the 21st century—had begun.
Almost as soon as the samples were analyzed, the CDC was able to isolate the novel strain and use it as the basis for an emergency vaccine. But flu-vaccine technology is decades old and clunky and the new virus did not cooperate, reproducing poorly and slowing the cumbersome process down. All summer and into the fall, anxious parents and doctors assailed pediatricians and drug manufacturers, begging for vaccine that did not exist yet. The first doses did not roll out to the public until October, after tens of thousands in the United States had been sickened and 60 children had died. The number of cases reported by doctors peaked in late October. By January, there was finally enough vaccine to protect everyone in the country who would typically get vaccinated, almost 120 million doses. But the public had lost interest, and more than a quarter of the hastily made vaccine—worth hundreds of millions of dollars—was destroyed.
The swine influenza of 2009 turned out not to be the grave danger that health authorities feared. Millions of people fell ill worldwide, but their illnesses were mild, for the most part. Between 151,700 and 575,400 people died—but while that seems like a large number, it was on a par with an average flu season. The worst impact was not to lives and health, but to the public’s trust in flu vaccines. The episode ended with health authorities making new efforts to fundamentally change the way flu shots are made and distributed.
And now they may have a glimmer of a chance.
**********
A Vaccine for All Seasons
To protect against future influenza epidemics, researchers are going beyond the usual shot in the arm. --Research by Sonya Maynard
In the last days of June this year, a phalanx of influenza scientists from around the world gathered in a sleek glass-walled conference space on a dead-end street in suburban Maryland. I was the only reporter in attendance at this invitation-only meeting, organized by the National Institutes of Health. The assembly had more in mind than simply speeding up vaccine delivery. Its goal was to examine whether flu shots could be completely reconceived, from a formula written and delivered fresh every year to one that could be given every ten years, or even once or twice in a lifetime: a universal vaccine.
Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, opened the meeting, which was titled “Pathway to a Universal Flu Vaccine.”
“Current seasonal flu vaccines are not consistently effective,” he told the roughly 175 attendees. “The measles, mumps and rubella vaccine is 97 percent effective; yellow fever vaccine is 99 percent effective. [Flu vaccine] can be as low as 10 percent.” In the flu season that ended in the spring of 2017, he said, the vaccine had prevented illness in just 42 percent of the people who took it.
Those numbers may come as a surprise, when you think of how aggressively public health encourages the flu vaccine. The CDC recommends that every U.S. resident who is 6 months or older and doesn’t have an allergy to any of the ingredients should receive the vaccine each flu season, and every year, manufacturers produce as many as 166 million doses to feed that demand. You cannot walk into a supermarket or a drugstore in the autumn without being urged to take the shot. Huge workplace campaigns ask employees to take it, and schools advertise the vaccine for kids who might infect newborns or vulnerable grandparents, as well as fall ill themselves.
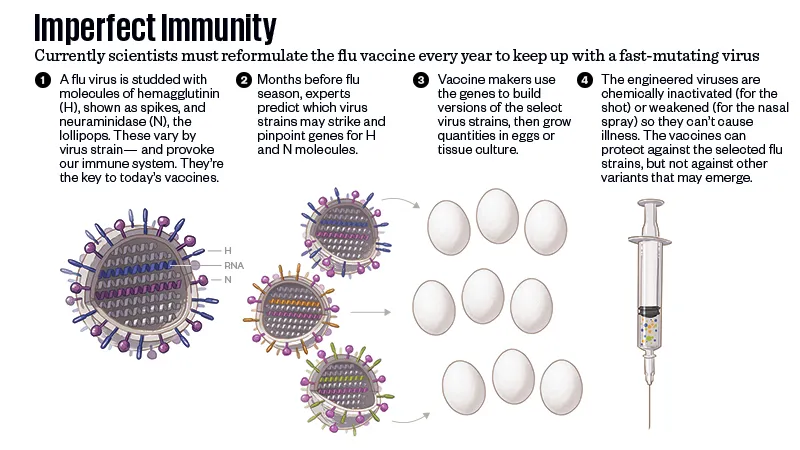
It’s precisely because of flu’s unpredictability that authorities push the flu vaccine so hard. The measles virus that circulates in the world now is the same as the one that existed 10 years ago, or 20 or 50. But flu changes from season to season, because as it reproduces, it makes constant small errors in its genetic code. The viruses flourish in the cold weather, cycling back and forth across the Equator each spring and fall. As a new flu season unspools, planners analyze the circulating viruses to predict what might happen when the disease heads toward the other pole again, and write a vaccine formula to match.
Flu vaccine manufacturing is a slow process. The viruses that planners select as best representing what might be coming—there are usually three, and in some formulas four—are inserted into a medium that will let them reproduce in large quantities. (Historically, vaccine developers used millions of fertilized chicken eggs, but now they sometimes incubate the viruses in lab-grown cells from animals or insects.) Then they deactivate the virus, for the injectable vaccine, or weaken it, for the nasal spray. It can take six months to grow enough virus and test and package a vaccine. In that time, flu’s restless mutability may send a season’s strain in a direction that no one expected, lessening the protection that planners hoped for when they wrote the vaccine formula half a year before.
According to the CDC, between 12,000 and 56,000 people die of flu every year just in the United States, and up to 710,000 more are made sick enough to be hospitalized. Those numbers comprise people who refuse the vaccine, and those who cannot take it because of allergies to one of its components. But they also include people who were vaccinated but ended up not being protected because the circulating virus didn’t match expectations.
That is the toll in average years, when the virus has altered itself just enough—“drifted” is the technical term—to require manufacturers to slightly adjust the previous year’s vaccine formula. But a few times a century, across unpredictable gaps of time, the virus doesn’t drift, but shifts, into a form so new that the existing vaccine is no use as a base for a new one, and prior infection provides no defense. When a flu such as that gets going, the result is a pandemic.
The 1918 flu was the mother of all flu pandemics. But there were pandemics as well in 1968 and 1957, which killed at least one million people each—and, based on historical accounts but with no microbiology to confirm them, in 1889, 1847, 1830, 1781, and as far back as an epidemic of “gasping oppression” in 1510. The flu virus was only identified in the lab in 1933, and the first vaccine was licensed in 1945.
“We need a better vaccine, for sure, that is broadly protective and has much longer-lasting durability,” says Dan Jernigan, the director of the CDC’s flu division, who represented the agency at the NIH meeting. “How far off that is, I can’t say.”
**********
If you could cross-section a flu virus, it would look roughly like a ball, studded with molecules that resemble spikes and mushrooms. The spikes are hemagglutinin, known as H or HA for short; the mushrooms are neuraminidase, known as N or NA. There are 18 subtypes of hemagglutinin and 11 subtypes of neuraminidase, and influenza A strains (the strains that cause pandemics) are named for the combinations of the two they harbor. The 1918 virus was an H1N1, 1957 was an H2N2, 1968 was an H3N2. (Within a given strain, such as H1N1, further mutations can occur over time, especially when an avian virus finds its way into other animals such as swine.)
Hemagglutinin is the part of the virus that allows it to bind to the cells in our lungs, to turn them into tiny factories for making more viruses. Because it’s on the surface of the virus, our immune systems react to hemagglutinin first. The problem is that the virus is constantly mutating. The antibodies we produce against this season’s hemagglutinin will not necessarily protect us against future strains of the flu.
But what if a vaccine could be made from a part of the virus that never changes?
“This is something that we’ve only been able to think about for perhaps the past five years,” says Peter Palese, the chair of microbiology at the Icahn School of Medicine at Mount Sinai in New York City. “Understanding viral immunology, and specifically the structure of hemagglutinins, has let us think about vaccine constructs that would elicit a broader immune response.”

Palese is one of the world’s most distinguished flu researchers, with a long list of publications and patents. The walls of his office at Mount Sinai, which looks toward the East River and the runways of LaGuardia Airport, are lined with framed awards and degrees earned and honorary, starting with his PhD from the University of Vienna in his native Austria. He has been studying flu for more than four decades, establishing the first genetic maps of influenza viruses and defining the mechanisms of antiviral drugs. He also pioneered a method of introducing mutations into the genome of influenza viruses, allowing us to understand how they cause illness.
Palese’s arrival at Mount Sinai in 1971 came just five years before a cluster of cases of flu occurred among military recruits at Fort Dix in New Jersey, a 75-mile drive from his lab. The cases were caused by a strain of swine flu; Palese was perfectly placed to watch the national panic as federal experts predicted a pandemic would spark from the anomalous strain, and formulated an emergency vaccine. Their prediction was wrong. There was no pandemic—but there was a simultaneous outbreak of temporary paralysis, called Guillain-Barré syndrome, in more than 450 people who received the shots. The vaccination campaign was called off amid chaos. The episode cast a pall over flu vaccine research for years afterward, while spotlighting the crucial need for a vaccine that didn’t need to be created fresh whenever a crisis threatened.
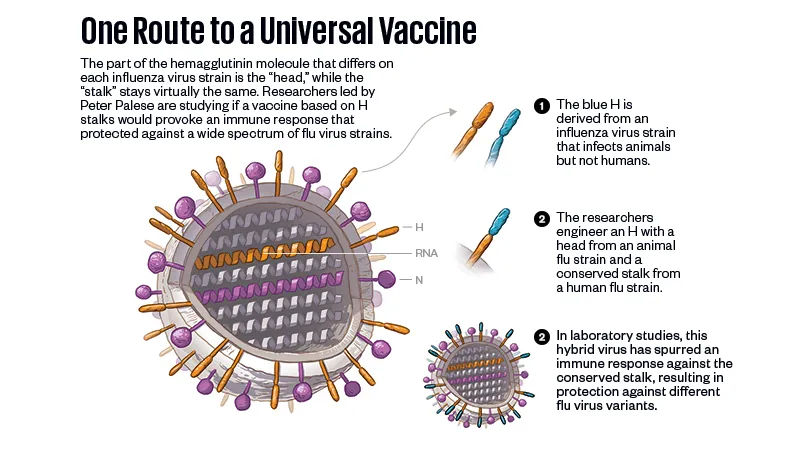
For decades, a universal formula seemed nearly inconceivable. Then, within one week in 2009, two sets of researchers announced that they had identified antibodies that attached not to the lollipop head of the hemagglutinin but to its sticklike stem. This was electrifying, because the stem of hemagglutinin is “conserved,” in technical language: It is substantially the same from strain to strain. The discoveries sparked hope that stem antibodies could defeat not just one virus strain, but many, and that turned out to be true. The research teams revealed that the antibodies they had found provided protection from a number of influenza virus strains.
But there was no obvious way to convert that hope into a vaccine. Stem antibodies are rare because the immune system so seldom has a chance to react to the stem; in its encounter with flu virus, it meets the hemagglutinin head first. To make the stem the basis of a vaccine strategy, researchers would have to perform some sort of surgery on hemagglutinins; in a maneuver like whacking a golf ball off a tee, they would have to move the molecules’ heads out of the way.
In the years since those discoveries, researchers have tried but failed to remove the head successfully: A beheaded stem simply falls apart, and antibodies won’t bind to it. There have also been promising achievements, methods of anchoring the stem of the hemagglutinin with engineered nanoparticles or with swapped-in amino acids.
Palese and his lab have developed a different strategy. In 2013, they removed the head of an H1 hemagglutinin and replaced it with the head of a hemagglutinin from a separate branch of the flu virus family tree—a strain that affected animals but not humans. (The researchers later developed a way to grow these particles from scratch, with the foreign heads already in place.) The substitution was meant to direct the immune system to skip past the new head as though it didn’t exist, generating antibodies to the stem instead. The stratagem worked. The chimeric hemagglutinin provoked an immune response and protected lab animals from infection. A Phase 1 trial has just started for human subjects.
“We have done it in mice, in guinea pigs, in ferrets—there it works wonderfully,” Palese said. “But mice are not men; ferrets are not humans. It really has to be tested in people.”
**********
In 1997, a research team at Walter Reed Army Medical Center announced it was bringing back to life the virus that caused the 1918 flu.
Scientists had never been able to explain what made that pandemic so vicious. It ended long before flu viruses were isolated in labs. Historical accounts testified to the rapid, dramatic way it killed its victims, but the virus itself seemed destined to remain a mystery. But at the end of the 20th century, researchers at the Armed Forces Institute of Pathology disclosed that they had found fragments of the virus in a long-stored autopsy sample, taken from a soldier who died in 1918.
No one in the tightknit world of influenza scientists had worked on flu research with this team of molecular pathologists. It was led by a pathologist, Jeffery K. Taubenberger, whose achievements included reassembling a measleslike virus that killed a pod of dolphins. Now, armed with the autopsy sample from the fallen soldier, the team received help from other virologists—and from a retired pathologist who went to Alaska on his own initiative to take tissues from an Inuit victim whose corpse had been frozen in the tundra for the past eight decades. In 2005, the Taubenberger group finished reconstructing the entire 1918 virus and extracting its genomic sequence. The astonishing achievement made headlines all over the world. “That Jurassic Park, Frankenstein thing of resurrecting a killer virus—you can see how that generated interest,” Taubenberger says. “But it wasn’t done just for the gee whiz factor.”

For scientists, Taubenberger’s work on the 1918 virus started to open the black box of what made it so virulent. It helped them better understand how influenza viruses adapt to humans, and what it might take to prevent modern-day pandemics.It is not easy to visit the NIH campus; it requires parking in a secure lot, passing through a line like an immigration check, shoving your bag through a scanner and having your picture taken for a temporary ID. To visit the scientist who resurrected the 1918 flu requires more effort yet. Cellphones are taken away and locked up—building rules permit no cameras—and Taubenberger himself must come to the lobby and swipe a badge to let you in. On the floor where he works, there are nested sets of locked doors, retina scanners, coded padlocks on the freezers and layers of sterilization systems. Together, they contain the threat represented by the reconstituted virus, and other deadly viruses that require high degrees of biocontainment.
When I visited, Taubenberger had just moved to a small, spare office that opened onto rows of laboratory benches and fume hoods and incubators. Most of his books and research papers were tidily heaped in boxes on the floor. A framed poster propped to one side advertised a performance of a string quartet he wrote more than two decades ago (“No. 2 in G Major”). Taubenberger plays the oboe, English horn, clarinet and piano, and he conducted the overture to his first operetta at George Mason University when he was 20 years old.
Now, at 56, Taubenberger is the chief of the viral pathogenesis and evolution section of the National Institute of Allergy and Infectious Diseases, the NIH agency that Fauci heads. But other flu vaccine researchers still see his background as unorthodox, and his approach is very different from Palese’s. “I didn’t try to turn out to be an anti-stalk guy,” he told me. “I think that immunity to stalk is likely to be important. I don’t think that it is the magic bullet other people are thinking.”
Taubenberger’s version of a universal formula hinges instead on what are called “virus-like particles,” VLPs for short. The FDA already has approved VLPs for vaccines against hepatitis B and HPV. Taubenberger’s group built on those models. To create their initial version of a universal vaccine, they used VLPs displaying hemagglutinins from four different strains of the flu that had caused past pandemics, including the one in 1918. They then combined the four kinds of VLPs into a “cocktail” vaccine, hoping it would provide broader protection than seasonal vaccines do.
The construct worked better than they expected. In mice, it provoked a protective immune response against strains carrying any of those four hemagglutinins—and also, to their surprise, against other strains that didn’t match the vaccine’s subtypes. Taubenberger is candid about the fact that he doesn’t yet understand how his vaccine invokes such broad immunity. “The question of how it works to protect all flu types,” he said, “is something we are still working on.”
If a flu vaccine could be made to protect against all forms of the virus, it would not only provide much better immunity but also change the whole process of how we administer flu shots. It would make it possible to give one vaccine, early in life, perhaps with periodic booster shots down the road. It would decompress the pressure to vaccinate the vulnerable in the short space of time before a new flu season begins.
Like Palese, Taubenberger would like to see a universal flu shot become part of the regular vaccination schedule. That would save more lives than we probably realize, he added. Though we think of pandemics as being the big killers, in the 100 years since 1918, they occupied only about six. “Except for 1918, there probably have been no pandemics in the 20th century, or early 21st century now, that have had impacts higher than really bad seasonal flu years,” he said. According to the CDC, the 2009 pandemic caused more than 12,000 deaths in the United States. “Seasonal flus,” said Taubenberger, “are right in that range every single year.”
**********
A month after the June meeting, I met Fauci in his NIH office. He is an immunologist, with a special interest in HIV—he assumed the directorship of NIAID in 1984, in the earliest days of the AIDS epidemic—and that gives him unique insight into the problems of achieving desperately needed vaccines. After all, it was in 1984 that then-Secretary of Health and Human Services Margaret Heckler declared that a vaccine against HIV could be achieved “in approximately two years.” It still has not.
Since the beginning of that epidemic, according to the World Health Organization, about 35 million people have died because of HIV infection. That is about one-third the estimated toll of the 1918 flu pandemic, and those numbers highlight how important a universal vaccine would be.
“There are still some scientific problems,” Fauci told me. “Can we really induce a response that truly is cross-protective between strains? I think the answer’s yes—but I can’t tell you we will get a truly universal influenza vaccine, because I’m not sure we’ve scientifically proven we can.” Still, he reiterated, “We have to stick with it. With a universal influenza vaccine, we could take pandemics off the table, instead of chasing our tails every ten years about a new bird flu or a new swine flu. Such a vaccine would also allow us to do better on seasonal flu, so that would be a twofer.”
For now, Palese and others continue to focus on inducing stem antibodies, while Taubenberger’s group keeps working on its cocktail approach, hoping to begin human trials in a year or so. Other groups are pursuing different strategies. One approach involves a protein called matrix 2, which is encoded on the influenza virus’ RNA and allows it to empty its contents into a cell. Another method focuses on activating T cells, which kill cells infected with the virus.
Whichever method turns out to be successful, and more than one could, it will face the same problem: A vaccine is not just science. It is also regulation, and manufacturing and marketing. In those realms, a universal flu vaccine faces challenges that are entirely separate from the scientific ones. The current, imperfect flu vaccine brings in more than $3 billion per year worldwide.
“The real challenge is that there is already an established, and very mature, private-sector enterprise producing flu vaccine that has in place a system of annual delivery that guarantees a certain amount of money,” said Michael Osterholm, the founder of the Center for Infectious Disease Research and Policy at the University of Minnesota. “How are you going to change that? Who is going to pay for that, given that the cost of research and development may mean the vaccine will be substantially more expensive than what we already have? What company will embrace that?”

In 2012, Osterholm’s organization released a comprehensive report calling for “game-changing” influenza vaccines. In that report, and in a book published earlier this year, Osterholm argued that merely producing new formulas in the lab cannot move flu vaccination forward. He envisions both a government-funded Manhattan Project and a philanthropic effort to support intensive research for a new vaccine.
Once that is achieved, he wants to see the public and private sectors make some financial guarantee to manufacturing companies that they will profit from switching to the new vaccine. “Until we do that,” Osterholm says, “flu vaccine is practically an orphan drug.” In other words, there’s little incentive for pharmaceutical companies to invest in research and development.
Other recent vaccine efforts haven’t faced the same challenges. Two years after Ebola ravaged West Africa, a team of scientists from the World Health Organization and the Guinea Ministry of Health produced a vaccine that protected 100 percent of the recipients from the infection. And more than a dozen companies are now racing to produce a vaccine against Zika virus, which invaded South America in 2015; a version could reach the market by next year. These efforts were monumental. But they can’t be compared with the quest for a universal flu vaccine.
The problem is that influenza is not like other diseases. It is not always as deadly as Ebola; it is not as novel as Zika. It is a disease so familiar that we use it as a synecdoche for other illnesses—we stay at home with “a flu” that is actually a cold, or are felled by a “stomach flu” that is actually a gastrointestinal bug. And influenza is caused by a virus so shape-shifting that we have never been able to anticipate which form it will take next. The difficulty of pursuing a universal vaccine for flu is not just the challenge of making new science. It is the challenge of reconceiving our relationship with a pathogen that is so close to us, we cannot see it clearly.