Can Virus Hunters Stop the Next Pandemic Before It Happens?
A global project is looking to animals to map the world’s disease hotspots. Are they going about it the right way?
:focal(689x250:690x251)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/e2/5a/e25ac109-cd4c-4edc-a1d6-62c4e86bc7fb/_eha3956_2-wr2.jpg)
Last summer, Dr. Kevin Olival joined a group of Indonesian hunters as they ventured deep into the mangrove forests of South Sulawesi island. The hunters were looking for roosting bats, mainly fruit bats and flying foxes—for them, a lucrative prize that can be shipped to villages in the north as part of the bushmeat trade. For Olival, the bats were a prize of a different sort.
Olival is a virus hunter. For more than 15 years, the ecologist and evolutionary biologist has scoured the globe for samples from animals that harbor some of the scariest undiscovered viruses as part of the global nonprofit EcoHealth Alliance. His goal: to find the next undiscovered virus in animals that harbors the ability to jump to humans and cause the next killer pandemic.
He and his team are in Indonesia for two weeks, swabbing feces, urine and saliva and taking blood samples from bats; freezing them in liquid nitrogen; and shipping them to an Indonesian laboratory for testing. EcoHealth Alliance is partnering with a larger collaboration known as USAID PREDICT, a $200 million global project aimed at detecting, preventing, and controlling infectious emerging diseases before they become full-blown pandemics.
The idea is fairly straightforward. If scientists can identify the places where viruses are most likely to jump from animals to humans, then they can warn people, get them to change any behaviors that increase risks, and contain any emerging infection. The difficulty is in the identification. That’s why Olival and others are trying to build an early warning system—one that’s still very much in its infancy.
"We're trying to improve the crystal ball, which is very murky," says Jonna Mazet, the global director of PREDICT and a professor of epidemiology at the University of California at Davis. The question is: is targeting animal vectors the best way to achieve that goal?
.....

Zoonotic viruses—those that jump from animals to humans, or vice versa—have caused some the world’s most devastating pandemics. Of the roughly 400 emerging infectious diseases that have been identified since 1940, more than 60 percent have animal origins. Bubonic plague originated in city rats. HIV/AIDS started as a virus in monkeys. Ebola found a home in bats before it jumped to humans, in an area of Guinea scientists had labeled a virus hotspot as early as 2008. The Spanish influenza pandemic of 1918, which racked up an unimaginable death toll of around 50 million people, has been traced back to birds.
Yet while it’s hard to imagine, a future zoonotic breakout could potentially be worse. "The world is not prepared,” says Dennis Carroll, director of the Global Health Security and Development Unit at USAID, over email, “to either mitigate the impact of an emerging threat or prevent its emergence—leaving us vulnerable to their consequences." Those consequences could include millions of lives lost, and billions of dollars in economic destruction.
Today, some believe the rate of emerging new diseases is rising. Studies find that modern factors like climate change, ecological degradation and population pressures may make it more likely that viruses jump from animals to humans. "We need to be better informed about future infectious disease threats before they emerge,” writes Caroll, “so that our technological countermeasures and our mitigation responses can be better tailored to the specifics of the threat in advance of its emergence."
In the meantime, PREDICT and partners like EcoHealth are beginning to piece together the most likely emerging threats.
…..
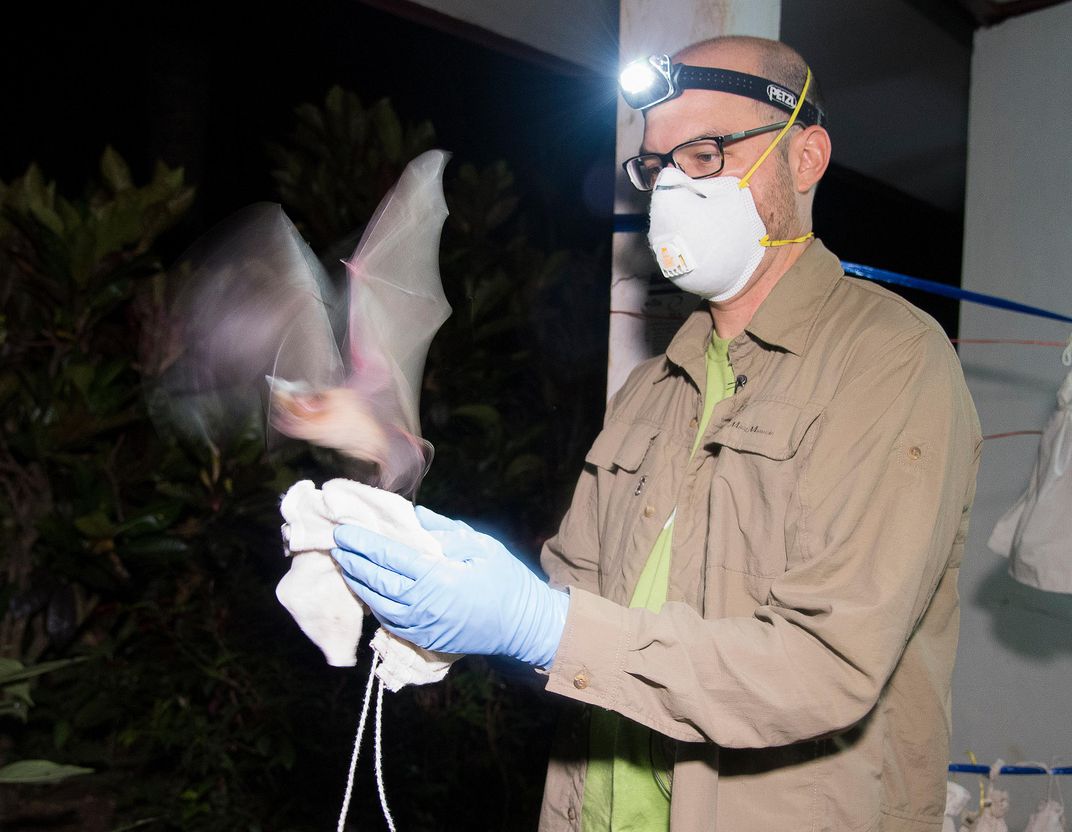
Places like Sulawesi, where roughly 500 tons of bats are killed and end up in the north, are primed to become the epicenter of the next pandemic. This mass movement of meat not only threatens bat conservation—the larger bat trade has contributed to population declines of bats across Southeast Asia—but also has the ability to spread infection to other parts of the country in no time. It’s a potent example of how globalization has created conditions for the likes of HIV/AIDS or Zika to spawn.
"Bush meat hunting, animal transport, direct contact,” says Olival. “It's a high-risk interface—exactly the type of place we are most interested in for the PREDICT project."
Bats carry a higher proportion of yet-to-be-identified viruses risky to humans than any other mammals. That fact has fascinated Olival since 2003, when he started researching the intersection of virus and animals following an outbreak of Nipah virus in Malaysia a few years earlier. His research on bats has connected him to some of the most frightening diseases of the times: Ebola, SARS, Marburg, Hendra, and likely MERS are killer viruses carried by these airborne mammals.
The samples Olival collected in Sulawesi were sent to an Indonesian lab, where they would be used in part to help create local resources in the hopes of making responses to emerging viruses nimbler. Yet while local labs are increasingly analyzing samples creating better surveillance on the ground, much of PREDICT’s work uncovering new viruses and creating a global database has been completed in Simon Anthony's laboratory at Columbia University's Mailman School of Public Health.
Anthony’s team examines and sequences more than 5,000 samples of blood and tissue annually. Many are from animals in the world's disease hot spots, places where humans and animals carrying viruses often come into dangerously close contact. At one point, he was credited with discovering 150 viruses; Stephen S. Morse, a former co-director of PREDICT and a professor of epidemiology at Columbia University, says Anthony has uncovered more new viruses than anyone.
"The end goal is to try and be better prepared, to try and prevent viruses from spilling over (into humans) in the first place," Anthony says. "That's a very complex and multi-layered prospect. We want to have some idea which ones are potentially dangerous and which ones are not ... We're literally at the start of doing that."
When he began working in 2008, Anthony’s team used to announce a new virus count at the beginning of each meeting. As their discoveries became more frequent, they had to abandon the ritual for practicality’s sake. The PREDICT team globally, he says, has found more than 1,000 new viruses spanning 20 countries.
Anthony spends most of his time staring at a computer screen, sequencing the genetic code of a virus. He then plugs that into an open-source database. If the code he has uncovered isn’t recognized, he knows he has discovered a new virus. "Your reward is looking at those results on those days and knowing you're the first person in the world to discover something," he adds. "That's what's awesome about this kind of work."
But right now, he says, there's no way to tell from the sequence whether a newly discovered virus can infect and thrive in human cells. That requires a series of physical experiments in the lab.
The initial five-year PREDICT study explored how to best collect data about viruses. The second five-year stage, which is funded for two more years, c has begun identifying high risk areas like Sulawesi, and whether humans are being infected by viruses in those places. Since 2014, PREDICT teams have sampled more than 26,000 animals and 1,700 people in 26 countries, mostly in Africa and Asia.
Smithsonian’s Global Health Program is the lead investigator testing animals and humans in Kenya and Myanmar, which were added to PREDICT three years ago. So far, the focus has been not only on sampling, but training local laboratory partners and creating a communications strategy for quickly disseminating information about risks, says Suzan Murray, a Smithsonian wildlife veterinariay medical officer and the program’s director.
“Our goal,” she says, “is to train ourselves out of a job.”
…..
PREDICT is not the first virus detective project. In 1915, the Rockefeller Foundation funded a virus hunt in developing countries that sought to research and eradicate yellow fever. During their surveys, they found a number of new viruses—including the Zika virus in Uganda in 1947, six decades before it jumped to humans. But PREDICT is the largest virus hunting effort underway today.
It’s also a proof of concept for something even more ambitious: the Global Virome Project. The proposed project, which has yet to be funded, aims to pre-empt pandemic threats by identifying and sequencing nearly half a million viruses that may spill over into humans.
The idea grew out of a meeting that Mazet attended at the Rockefeller Foundation's Bellagio Conference Center last August. At the meeting, Mazet says she was shocked at how much enthusiasm World Health Organization leaders showed for taking on such an ambitious and costly project. "Some big thought leaders said there's nothing more important we could do (to protect human lives)," she adds. "This and climate change. These are the biggest threats to our society and we need to deal with this."
Whether funding will emerge for the 10-year project is unclear. The cost has been estimated as high as $3.4 billion but proponents like Mazet say it would pay for itself many times if it stopped even one pandemic.
In the meantime, PREDICT is beginning to piece together a composite picture of where to look for emerging viruses. "We have this mosaic of different studies from all over the world," Mazet says. "Our team and others have done a great job of making a beautiful picture out of that mosaic, but it is still just a mosaic of this haphazard activity."
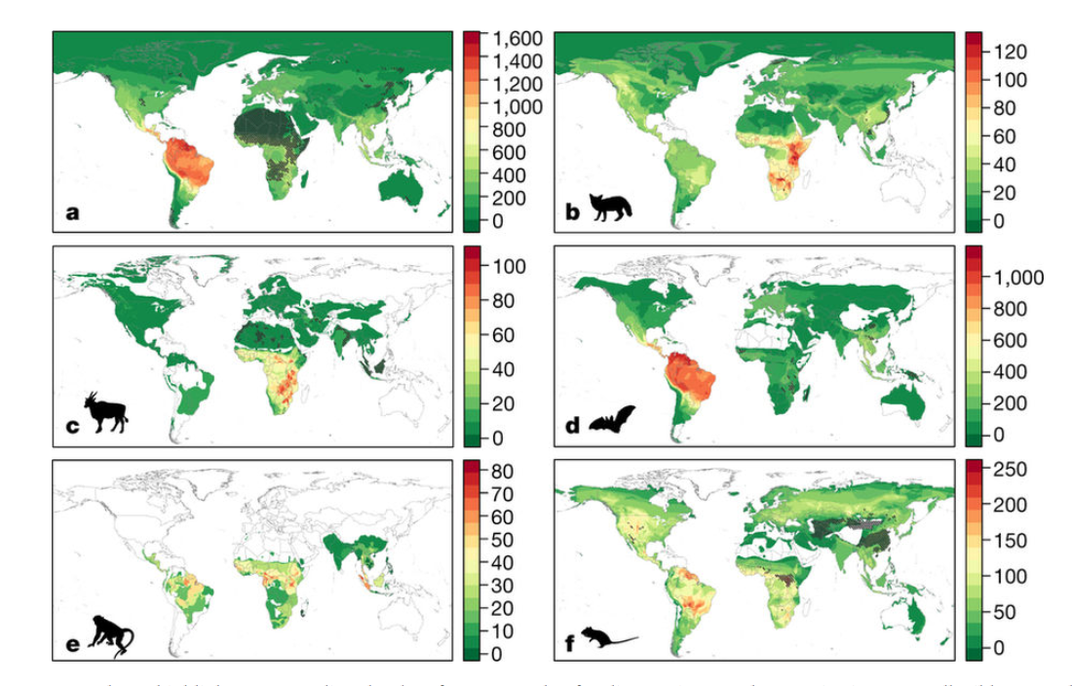
One example is a study Olival and his colleagues at EcoHealth published last year, which included detailed maps highlighting areas for as-yet-undiscovered viruses that could emerge in humans. The maps are extrapolations created through computer modeling, so they don't have a high level of granularity, he notes, but they do prioritize regions of the world and ecosystem types that are at high risk, places to watch.
“The holy grail in pandemic prevention is to understand where the next zoonotic virus is likely to emerge and from what species,” Olival says. “Our study provides the first ever predictive map of where these undiscovered zoonoses can be found across the world. This information is critical to prioritize surveillance to identify and stop the next pandemic.”
The maps reveal hot spots for bats in South and Central America and parts of Asia, and for primates in tropical Central America, Africa and Southeast Asia. Tthe greatest potential for future viral outbreaks comes from bats in northern South America, carnivores in East Africa, primates in tropical Central America, Africa, and southeast Asia, and hooved mammals (cattle, sheep, camels, deer) in east and central Africa.
Ultimately, the goal is to be in front of a pandemic—rather than chasing it—by knowing early on what viruses are out there and working with local communities to help them understand the risks. "Having that knowledge in the community allows people to make different choices," Mazet says. "That's what we want with viruses. We have it for driving cars. We have it for most bacterial issues. But we know almost nothing about viruses. They're, frankly, what causes epidemics and pandemics. "
…..
As an example of how changing behavior can dramatically reduced risk, Olival and Mazet point to Bangladesh. Until recently, the country suffered from regular outbreaks of the deadly Nipah virus, which has killed as many as 50 people a year since 2001. In 2016, there were no reported outbreaks of the disease.
Government officials credited an education campaign for this reprieve. Olival credits the disgust factor.
When he traveled to the country, he found that locals drink raw date palm sap collected from trees where bats roost. Bats attracted to the sap had often contaminated it with their urine or saliva. By mounting infrared motion-sensing cameras in the trees, researchers showed bats licking the sap and urinating from the trees. They then appealed to residents to cover pots with a bamboo skirt and to boil sap.
Moreover, analysis of those bats revealed they carried more than 50 newly discovered viruses, says Olival. "This low-tech solution not only can stop Nipah from emerging, but also prevent 50 other viruses from jumping into people," he says. "It would cost an enormous amount of money to create vaccines versus a few dollars for a bamboo skirt."
When Anthony looks at the emergence of Nipah, he sees another question to explore. Researchers have found other viruses related to Nipah, he notes, but none of them have infected people. "Why did Nipah spill over and not these other viruses? What's special about Nipah that these other viruses don't have that allowed Nipah to emerge as a human pathogen?” he asks. “We don't know the answer yet, but these are the questions we can hope to start to get a handle on."
…..
Not everyone thinks that discovering viruses and their hotspots is the best way to prevent pandemics. Dr. Robert B. Tesh, a virologist at the University of Texas Medical Branch, says we don't understand enough about zoonotic viruses to create predictive models. “A lot of the stuff they produce is hype," he says, referring to PREDICT’s work. "It's more PR than science."
Tesh doesn't think you can predict the next outbreak for two main reasons. First, viruses like Zika and West Nile aren’t actually new; they were transported to new areas and then spilled over. "I don't think anybody could have predicted that," he says.
Second, many of these are reassortment viruses that mutate quickly. No amount of discovery can prepare for that. "Some die out and don't go anywhere," Tesh notes. "Others adapt to new hosts and go on."
He points to a recent study about West Nile virus, which is transmitted by mosquitoes. The study outlined numerous factors that go into that go into whether and where an outbreak will occur, including land use, climate, mosquito genotype and the microbiomes of those mosquitoes. “Given these variables and how little we really understand them, people who claim they can predict what will happen … are fooling themselves and the funding agency,” he says.
Tesh believes that in many cases—as with SARS and MERS, which pop in and out of humans long before they are noticed—human surveillance is the way to go. For example, the U.S. Centers for Disease Control (CDC) has long employed a surveillance project at six hospitals in Uganda. When a child comes in with an unexplained fever, doctors draw his or her blood. They test the sample for bacterial causes as well as viruses, creating an early warning system locally.
Dr. Ron Rosenberg, associate director for science at the CDC’s Division of Vector-Borne Diseases, declined to comment specifically on projects like PREDICT. But like Tesh, he said he believes the focus should be on identifying viruses in humans.
"In general, I think the best sentinels for discovering new viruses are humans, not animals," says Rosenberg, who edits the CDC journal Emerging Infectious Diseases. "The reason I say that is we don't really have a way of predicting whether a virus that we find in an animal ... will infect humans. There's no magic bullet. There's no secret key. There's no way we can look at the genome and say it has this gene and it's one nucleotide away from infecting humans."
That doesn’t stop PREDICT and other groups from trying. For them, the key to nipping species-jumping viruses in the bud is getting a baseline for what’s out there. Mazet compares the situation to early weather forecasters, who had decades of clean data for their models. Public health officials looking to prevent the next pandemic, she says, are flying blind by comparison.
"The biggest challenge for PREDICT right now 100 percent is that lack of information," she says. "We need that century of data (like weather forecasters have) and we don't have the time. We could lose cultures and societies if we wait 100 years to collect it."
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/jim-morrison-240.jpg)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/jim-morrison-240.jpg)