How Scientists Use Climate Models to Predict Mosquito-Borne Disease Outbreaks
The ebb and flow of rainy seasons corresponds with the hatching of millions of mosquitoes—and the spread of diseases they carry
:focal(942x609:943x610)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/8f/e8/8fe81f49-8f5f-4297-a676-f8782cd7ee0b/istock-157292773.jpg)
Few natural phenomena pose a greater threat to humans than a swarm of mosquitoes erupting from a cluster of soil-lodged eggs. These bloodthirsty menaces can carry a host of diseases, such as Zika, West Nile and malaria, making mosquitoes the world’s deadliest animals.
Mosquito-borne diseases threaten billions of people, and while the diseases vary in biology and geography, most, if not all, are exacerbated by climate change. Scientists predict that a warming world will invite the spread of more mosquitoes, and more illness, threatening a billion more people over the next 60 years. But long-term predictions are hard to act on, and public health experts believe short-term forecasts could better kick-start programs to save people’s lives today.
For the last 20 years, scientists studying weather patterns have pieced together how real-time data can help predict mosquito-borne disease outbreaks weeks or even months before the insects emerge from the ground. These tools may provide a mechanism to prevent millions of deaths, tracking monsoons and other rain cycles to forecast mosquito hatching events.
“I think the issues are kind of undeniable,” says Juli Trtanj, climate and health lead at the National Oceanic and Atmospheric Administration (NOAA). Warmer temperatures, more frequent droughts, devastating wildfires and powerful hurricanes have significant implications for public health—and the seasonal birth of millions of mosquitoes, tied to weather patterns, is perhaps the greatest public health risk of them all. “The fact is, we can observe it. We can predict it. And we need to do something about it.”
Outbreaks can happen in the blink of an eye. The Zika virus, carried by Aedes aegypti mosquitoes, infected over a million people in 2015 alone. Annual outbreaks of the debilitating chikungunya virus often affect millions as well. In East Africa, different species of Aedes mosquito (Aedes mcintoshi and Aedes ochraceus) threaten humans and livestock with seasonal outbreaks of Rift Valley Fever (RFV).
Toward the end of the rainy season, these mosquitoes lay their eggs in shallow grasslands, or dambos. When months of heavy rain flood the area the following year, those eggs begin hatching in batches, maintaining a constant stream of millions of potential disease carriers.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/a2/27/a227f2ae-6e17-44ea-a2e3-fff998b50a43/gettyimages-154727723.jpg)
To Assaf Anyamba, a research scientist at NASA’s earth sciences division, the rainfall-driven outbreaks have one primary culprit: the El Niño climate pattern. In 1997, Anyamba began studying how the warm cycle of El Niño (and cool cycle of La Niña) might relate to surges in mosquito-borne disease.
He and his team—a collaboration including NASA, NOAA, the Department of Defense and the Department of Agriculture—gathered mountains of data. They tracked surface temperatures on land and sea, followed expected climate patterns and weather observations, and used satellite images to calculate rainfall (vibrant green vegetation is a clear sign of well-watered land). All these metrics were compiled into a single tool which could pinpoint regions at risk of Rift Valley Fever outbreaks. In 2006, the RVF Monitor made its first prediction.
“Nothing like this had ever been attempted before, just to go out on a limb and issue a prediction,” Anyamba says. Based on the tool’s detection of strong El Niño conditions, the United States issued a warning to East African countries of the high risk for RVF in September 2006. “It was a very courageous statement from us, but we thought we were on to something.”
As it turns out, they were on to something. By November of that year, scientists in the region confirmed the virus. The team’s tool successfully predicted the disease months before it appeared. Anyamba attributes part of the success to solid science and the other part to strong international relationships. Regional governments and the international community mobilized life-saving resources two months before they would have otherwise responded to the impending outbreak.
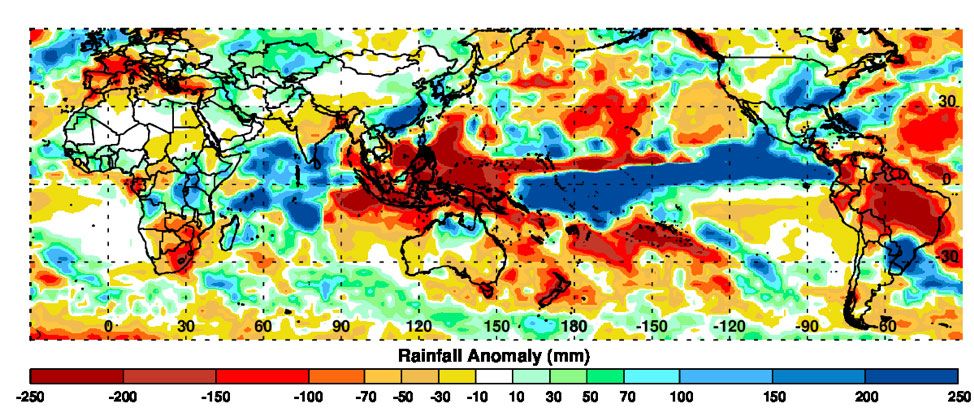
The research group continued to refine their mosquito-prediction tool while international relationships in the public health world developed further. Anyamba says the 2006 prediction was a successful “proof of concept,” but that they had their sights set on the global scale.
In 2014 and 2015, the team detected what would become one of the three strongest El Niño systems since 1950. They issued warnings globally, sounding the alarm for diseases such as malaria in Bangladesh, RVF in East Africa, and dengue and Zika in Brazil (where the high temperatures and droughts caused by El Niño in the Western Hemisphere can spark outbreaks).
For RVF, seven agencies came together to issue a first-ever “Emerging Health Risk Notification” based on the new model. “If we do this right, no one will hear about a Rift Valley Fever outbreak in 2016, because there won’t be one,” Trtanj said at the time.
The dambos flooded. The mosquitoes emerged. But humans and livestock were spared in East Africa. According to Anyamba, governments were proactive about animal vaccinations and outreach. “They took the message seriously,” he says. “This tells you, when bureaucracies uptake information in a timely manner, what can happen.”
To the west, 105 cases of RVF were detected in Niger, resulting in 28 deaths. The differences in disease prevention are likely attributable to distinct government resources and responses.

Trtanj emphasizes the importance of international partnerships. “It’s about building trust,” she says. Being confident in the science is one thing, but it takes years to develop the essential institutional relationships needed to turn science into action. Public education campaigns can be vital, too. In Kenya, the WHO funded radio broadcasts to alert local populations and caution against meat from sick livestock. Still, they believe constant disease surveillance and reporting can be improved.
According to the World Health Organization, more than half of the world’s 7.5 billion people are at risk of mosquito-borne diseases. Many of these diseases involve the same genus of mosquito. Both Anyamba and Trtanj envision using climate to alleviate this enormous global burden.
“The whole idea here is not actually that you’re going to be able to eliminate disease completely,” Anyamba says. “But it’s basically being able to manage and minimize.”
For the last three years, Anyamba’s team has shifted focus to predicting diseases from the Aedes aegypti mosquitoes which transmit Zika, dengue and chikungunya. They are using artificial intelligence to absorb data from satellite images, climate and weather observations, population density and real-time outbreak reports—all to pinpoint regions at risk of chikungunya outbreak. An app called CHIKRisk is being developed in partnership with the DoD’s Defense Threat Reduction Agency for public release later this year.
“There is no reason with the data, observation and modeling capacity we have in the world that we are still surprised by disease outbreak,” Trtanj says. “We should know better. We can do better. We should not still be caught flat-footed.”