Breaking Down the Two Tests That Could Help Contain the COVID-19 Pandemic
One detects an active infection; another signals that the virus has already left the body. Both are critical for tracking the spread of disease
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/16/21/1621c5ba-39bb-465a-ac57-981c695cb639/gettyimages-1215680441.jpg)
The new coronavirus, SARS-CoV-2, has proved to be extremely stealthy, often spreading without the obvious hallmark of symptoms. But no pathogen is truly invisible. When deployed at the right time and in enough individuals, modern molecular tests can guide treatments, map out how quickly and where a disease is spreading and pinpoint the people a pathogen has already touched.
Amidst a slew of shortages and logistical hurdles, American researchers are now slowly rolling out two crucial and very different tests to fight the COVID-19 pandemic: one that can detect an ongoing SARS-CoV-2 infection and another that can tell if the pathogen already passed through the body.
These two tests aren’t interchangeable, but they are complementary—and together, they’re likely to play a crucial role in giving health workers and the public the information they need to contain and end this pandemic.
“Testing matters from a global and public health standpoint,” says Jasmine Marcelin, an infectious disease physician at the University of Nebraska Medical Center. “This [outbreak] is moving so rapidly. If we don’t have a good understanding of how many people are affected, we will not be able to effectively curb the spread of disease.”
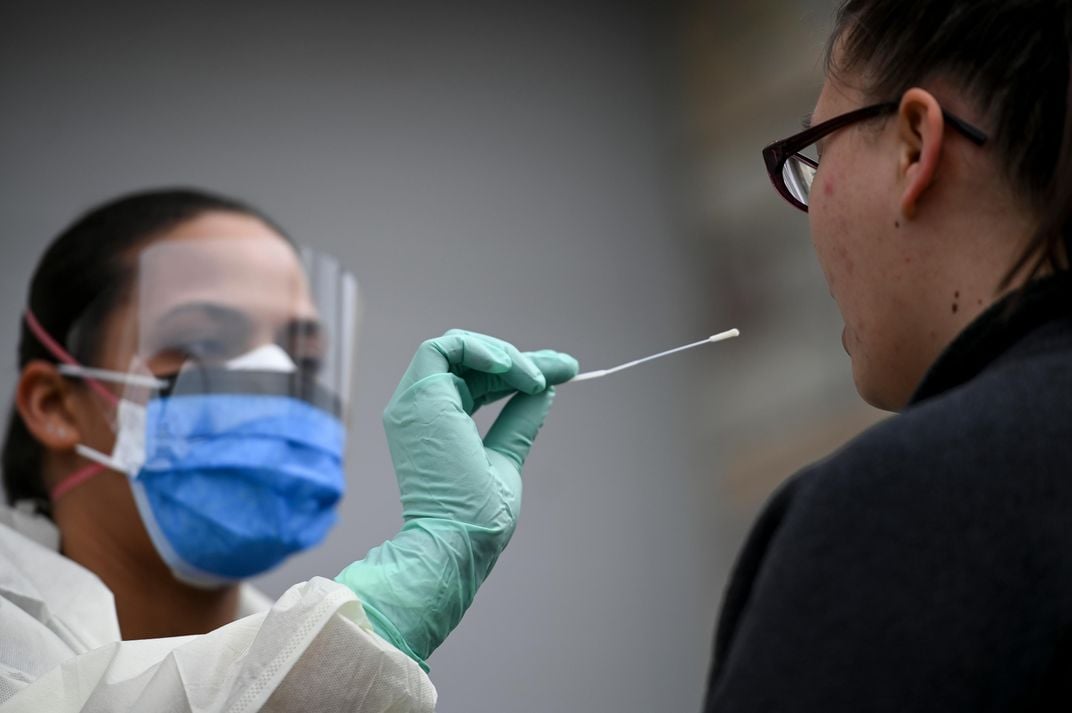
The viral swab test: Catching coronavirus red-handed
The first test detects an active infection by searching for SARS-CoV-2 genetic material in a patient’s airway, where the virus is most commonly found. This assessment serves two purposes: diagnosing the sick so treatments can be administered and alerting those at risk of spreading COVID-19 to others, Marcelin says.
To conduct the test, researchers first send a long, thin swab deep into the nose to collect fluid and cells from the nasopharynx, a cavity that sits just above the throat. They then extract viral genetic information from the end of the swab and prepare it for reverse transcription polymerase chain reaction (RT-PCR), a technique used in laboratories to amplify very small quantities of DNA.

SARS-CoV-2 stores its genetic information in RNA, so its genome must first be transcribed into DNA. The test then hunts for snippets of the SARS-CoV-2 genome by using tiny probes that will only bind to the DNA version of the virus’ genetic material. If the probes find their target, the DNA will get copied many times over; if no viral genome is present, the molecular Xeroxing won’t occur.
Built into the copying procedure is a fluorescent dye that lights up when it binds to DNA. When more copies of DNA are produced, the sample will glow more intensely and quickly. If the chemical reaction lights up after a few dozen cycles, the test is considered positive, suggesting the patient is infected with SARS-CoV-2.
While this test is considered very accurate in laboratory settings, errors can occur, says Alexander McAdam, director of the infectious diseases diagnostics laboratory at Boston Children’s Hospital. For example, contamination between samples in a lab may occasionally lead to a false positive result, which incorrectly indicates the virus has infected a patient who’s pathogen-free. Perhaps more troubling are false negatives, which erroneously reassure people they’re healthy when they aren’t, potentially hastening the spread of disease. False negatives can happen when swabs aren’t taken thoroughly enough or performed before the virus has replicated to high enough levels in the body.
Even perfectly performed tests have their limitations. For now, doctors can’t use positive results to forecast how a patient will weather COVID-19, says Akiko Iwasaki, a virologist and immunologist at Yale University. Some infected individuals will need to seek treatment for a serious illness, while many others may have only mild symptoms that don’t disrupt daily life.
Either way, viruses don’t need symptoms to spread. The mere presence of SARS-CoV-2 in the airway is reason enough to self-isolate so the infection doesn’t pass on to others—especially people more vulnerable than the patient.
“Isolating yourself will protect your family, and the rest of society, from you,” says Eric Rubin, an infectious disease researcher and clinician at Harvard’s School of Public Health and editor-in-chief of the New England Journal of Medicine.
The antibody blood test: Interrogating witnesses
A second type of test, which samples blood rather than airway secretions, is gaining traction worldwide. These assessments, sometimes called serological tests, detect antibodies, the Y-shaped immune molecules the body manufactures after it detects a specific microbe. Unlike viral swab tests, blood-based tests can’t reliably reveal the presence of a pathogen. Instead, they tell clinicians that a dangerous germ has recently passed through the body but has already vacated the premises. That makes them most useful for people who suspect their encounters with SARS-CoV-2 are already in the rearview mirror, Rubin says.
Much of the difference between the two types of tests comes down to timing. Antibodies don’t appear in large numbers for several days, or sometimes even weeks, after an infection begins. With relatively short-term diseases like COVID-19, the virus and the antibodies meant to thwart it may overlap by no more than a few days.
If the swab test is like catching an invader red-handed, the antibody blood test is akin to interrogating eyewitnesses after a break-in. But by checking for immune molecules produced by the body, antibody tests have the potential to do something that swab tests can’t. “Antibodies tell you that you’ve been exposed and have mounted an immune response,” Iwasaki says. For most diseases, these immune responses help protect patients from future infections by the same pathogen—a tantalizing possibility researchers are now actively investigating for SARS-CoV-2.
Blood tests are less direct than swab tests because antibodies can’t be read like a genome. Instead, they must be baited with something that resembles their target germ. In the case of SARS-CoV-2, that molecular lure is usually a synthetic version of the spike protein that sits on the virus’s surface and helps it latch onto and enter cells in the human airway.
In one version of the test, researchers attach the protein probe to the bottom of a plate and then expose it to a sample of a patient’s serum—the liquid portion of blood that contains antibodies. They then add a third ingredient: a fluorescent protein that lights up when it detects the patient’s antibodies. The more the plate glows, the more antibody is present.
Some blood-based tests can also indicate how recently SARS-CoV-2 infected a patient by distinguishing between the types of antibodies in their sample. These assessments measure the relative levels of two classes of immune molecules: short-lived IgM, the first antibody variant produced in response to an infection, and IgG, a later-arriving class of antibody that mounts the brunt of the attacks on disease-causing invaders. In broad strokes, individuals with more IgM likely had the virus in their bodies within the last few days—and may even be at the tail end of an ongoing infection—while those with more IgG tend to be further out from an active illness.
Blood tests are very good at what they are meant to do: telling doctors that a patient has produced antibodies, McAdam says. But they don’t show how well those antibodies are working. Some antibodies capable of producing a positive result on a test, for instance, may not actually protect a person against an actual virus.
The quantity of antibodies the body manufactures may matter just as much as the quality of those molecules’ virus-fighting skills. Even if a person can make antibodies that effectively attack SARS-CoV-2, scientists aren’t sure how many are needed to keep a person safe. The answers could vary wildly from person to person since factors like age and genetics have a big influence on an individual’s immune response. Until that protective threshold is better understood, antibodies alone can’t guarantee a person’s immune status.
Although antibodies are usually beneficial, they can sometimes inadvertently play a role in exacerbating disease, Iwasaki says. Hyperactive immune responses that damage healthy cells alongside infected ones are thought to contribute to many severe COVID-19 cases, and could, in theory, involve a strong antibody-based response.
“We just don’t know what type of immune response confers protective immunity, and what leads to devastating disease,” Iwasaki says.
In all, researchers remain unsure about whether people who have recovered from COVID-19 are fully protected against subsequent infections, Marcelin says. Early studies have suggested immunity against the new coronavirus is likely. But the world has only known about the virus for a few months, so experts don’t know whether all patients become immune after they’ve encountered SARS-CoV-2, or how long that protection might last.
Still, if antibody responses to SARS-CoV-2 work “the way we think they do,” positive results from blood tests could carry immense promise, Rubin says. The more researchers learn about the immune response to the new coronavirus, the more important these tests could become in ending the pandemic. If a patient who has fought off the pathogen is no longer vulnerable to infection, they would become “a very valuable person,” Rubin says. Recovered, immune individuals could be among the first cleared to reunite with friends and family, return to work or care for the sick.
A powerful combination
In an ideal world, everyone would have access to both types of tests to get a full picture of their infection status, past and present, Iwasaki says. But in the United States, tests remain in short supply.
At the start of the outbreak, most laboratories didn’t have clearance from the FDA to develop their own tests, saddling government agencies such as the CDC with the brunt of the work, McAdam says. The agency’s first batch of tests was faulty, and by the time more research facilities could enter the fray, COVID-19 had swept across the nation. In the weeks since, manufacturers have been forced to play a frantic game of catchup that’s now being further stymied by a shortage of nasopharyngeal swabs, McAdam says.
Given this limited capacity, health care workers should be among the first people tested, Iwasaki says. Those with active infections could then seek treatment, keeping both them and their patients out of harm’s way, while those with antibodies in their blood might be able to care for the sick under less risk.
Vulnerable populations, including the elderly and immunocompromised, represent another priority group for testing, Iwasaki adds. Troublingly, many of these individuals live in low-income and minority communities—some of the very places that have yet to see widespread testing and treatment, Marcelin says.
If more supplies and personnel were available, viral swab tests could be extended to the close contacts of infected individuals to help track the spread of infection through the population at large, Rubin says. Widespread testing beyond those showing symptoms would help researchers identify individuals who may be unknowingly spreading the virus, which is crucial to tracking and containing the spread of disease.
Similar surveys could be done with antibody blood tests, which will increase in importance as the pandemic evolves and the number of known cases continues to grow. Recovered individuals will no longer carry the virus, but their bodies should harbor antibodies that commemorate past illness. Finding these patients could help researchers acquire a clearer picture of where SARS-CoV-2 has been and how often infection leads to death.
However, as the number of confirmed COVID-19 cases in the United States surges higher into the hundreds of thousands, researchers and clinicians are no longer in a position to consider ideal scenarios. With supplies for swab tests running low, some in the healthcare industry are now trying to swap in blood tests as an emergency replacement.
This diagnostic substitution is “very unusual,” McAdam says. Because the antibody blood test is designed to check for an immune response that’s most prominent after an infection has run its course, it could miss new infections. If a patient’s blood is tested too soon, antibodies may not yet be present, even if the virus is already replicating in their bodies—something that only the swab test would catch.
Antibody tests are excellent at the job they’re intended for, McAdam says. Used in place of another assessment, however, they may not provide the information clinicians, researchers and patients need most.
Still, McAdam says, in the dire situation we’re in, a less-than-ideal test “is better than nothing,” especially for patients with symptoms who have likely already had the virus in their bodies for several days or weeks.
Tests alone won’t solve this global crisis, McAdam says; they’re a guide for infection control measures, not a replacement for them.
“This is a very serious illness,” McAdam says. “People should have the best understanding … of what to expect from this disease.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)



/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)