The Benefits of Probiotics Might Not Be So Clear Cut
An individual’s natural gut bacteria determine whether the so-called dietary supplements help or do nothing at all
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/69/68/6968028a-a767-4859-a6e2-9529f13e429b/istock-683402568.jpg)
From pickles and candy bars to pills and protein powders, probiotics are touted as a health boon in all flavors of foodstuffs. Consuming these beneficial bacteria can bolster the gut’s microbiome, allegedly enhancing everything from digestion to brain function. But regardless of what shape or size these probiotic supplements come in, they appear to have one thing in common—many people simply don’t benefit from them, and in some specific cases, they may actually do harm.
A pair of studies published today in the journal Cell examines probiotic dietary supplements to determine if the supposed wonder bacteria actually provide the kind of benefits that have been claimed. The results paint a more complicated picture, and they are likely to rankle many among the millions who swear by probiotic supplements.
Senior author Eran Elinav, an immunologist at the Weizmann Institute of Science in Israel, and colleagues found that many people’s gastrointestinal tracts reject generic probiotics before they can get to work. Even worse, Elinav’s team found that microbial competition from off-the-shelf probiotics can prevent natural gut bacteria from reestablishing themselves after being wiped out by antibiotic drugs.
“I think our findings call for a fundamental change from the currently utilized one-size-fits-all paradigm, in which we go to the supermarket and buy a formulation of probiotics which is designed by some company, to a new method which is personalized,” Elinav says. “By measuring people in a data-driven way, one would be much better able to harness different probiotic combinations in different clinical contexts.”
The studies certainly aren’t the first to question how effective generalized probiotic supplements really are. They do, however, offer an unprecedented look at how the supplemental bacteria populated (or didn’t) throughout the gut.
Still, the results aren’t likely to slow the growing interest in bacterial supplements. Probiotics have been around since Ilya Metchnikoff drank cholera back in 1892, but their popularity has soared in recent years, as has the number of doctors recommending their use. A recent study found that 60 percent of physicians had recommended probiotic foods or supplements, and a National Health Interview Survey showed that nearly 4 million Americans used probiotics in 2012—a number that has quadrupled since 2007.
Elinav’s group isn’t claiming that probiotic supplements don’t carry heavy doses of beneficial gut bacteria. In fact, the studies confirm that they do. Because many probiotics are sold as dietary supplements, and thus aren’t subject to approval and regulation by many national drug agencies, including the U.S. Food and Drug Administration, the team first set out to ensure that the probiotic supplements in the study actually contained the 11 main strains they were supposed to deliver.
“All those strains were present and viable to consumption and beyond, following the passage through the GI tract, and even in stool, and they were still viable,” Elinav says.
But uncovering what impact these strains of bacteria have on the people who consume them required more digging, poking through patient’s stool and even inside their guts.
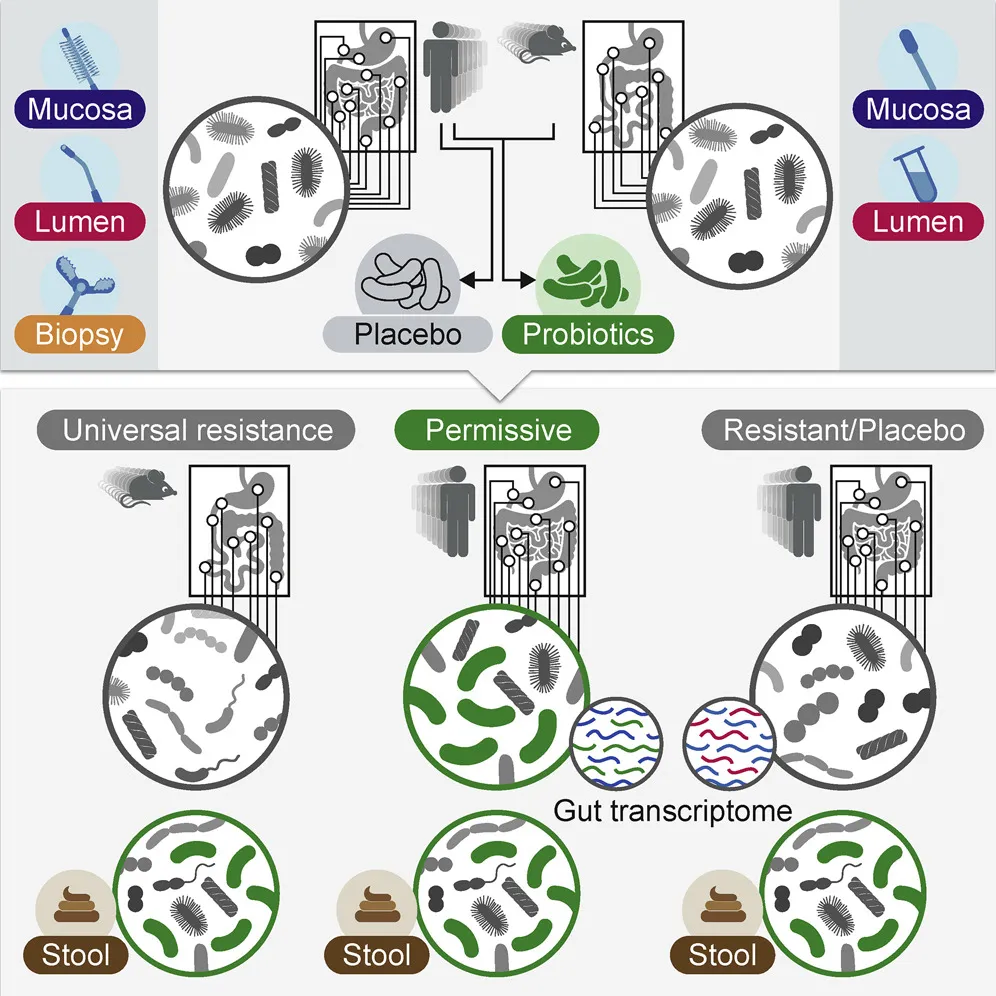
The authors set out to directly measure gut colonization by first finding 25 volunteers to undergo upper endoscopies and colonoscopies to map their baseline microbiomes in different parts of the gut. “Nobody has done anything quite like this before,” says Matthew Ciorba, a gastroenterologist at Washington University in Saint Louis School of Medicine unaffiliated with the study. “This takes some devoted volunteers and some very convincing researchers to get this done.”
Some of the volunteers took generic probiotics, and others a placebo, before undergoing the same procedures two months later. This truly insider’s look at the gut microbiome showed some people were “persisters,” whose guts were successfully colonized by off-the-shelf probiotics, while others, called “resisters,” expelled them before they could become established. The research suggests two reasons for the variability in the natural response of different gastrointestinal tracts to probiotics.
First and foremost is each person’s indigenous microbiome, or the unique assembly of gut bacteria that helps dictate which new strains will or won’t be able to join the party. The authors took gut microbiomes from resistant and persistent humans alike and transferred them into germ-free mice, which had no microbiome of their own. All the mice were then given the same probiotic preparation.
“We were quite surprised to see that the mice that harbored the resistant microbiome resisted the probiotics that were given to them, while mice that were given the permissive microbiome allowed much more of the probiotics to colonize their gastrointestinal tract,” Elinav explains. “This provides evidence that the microbiome contributes to a given person’s resistance or permissiveness to given probiotics.”
The second factor affecting an individual’s response to probiotics was each host’s gene expression profile. Before the probiotics were administered, volunteers who ended up being resistant were shown to have a unique gene signature in their guts—specifically, a more activated state of autoimmune response than those who were permissive to the supplements.
“So it’s probably a combination of the indigenous microbiome and the human immune system profile that team up to determine a person’s specific state of resistance or colonization to probiotics,” Elinav says. These factors were so clear that the team even found that they could predict whether an individual would be resistant or permissive by looking at their baseline microbiome and gut gene expression profile.
This unusual in situ gastrointestinal tract sampling also turned out to be key, because in a number of cases the microbiota composition found in a patient’s stool was only partially correlated with what was found inside the gut. In other words, simply using stool samples as a proxy can be misleading.
Emma Allen-Vercoe, a microbiologist at the University of Guelph who was not involved in the research, says consumers should be aware of probiotic limitations even if they happen to be the type of persisters who are more receptive to off-the-shelf probiotics.
“If you look on the side of any probiotic it will list the number of billions of CFU [colony forming units] and, wow, a billion sounds like a big number. But what you need to know is that there are trillions of microbes in the human gut. So what you’re putting in, and what’s surviving is fairly small in comparison,” Allen-Vercoe says. “Yes, these things do have the capacity to multiply, and some are better than others at doing that, but still what you’re putting in is a drop in the ocean.”
Elinav’s second study may be the first to suggest that, in specific cases, probiotic supplements might do worse than nothing at all—they might actually cause harm.
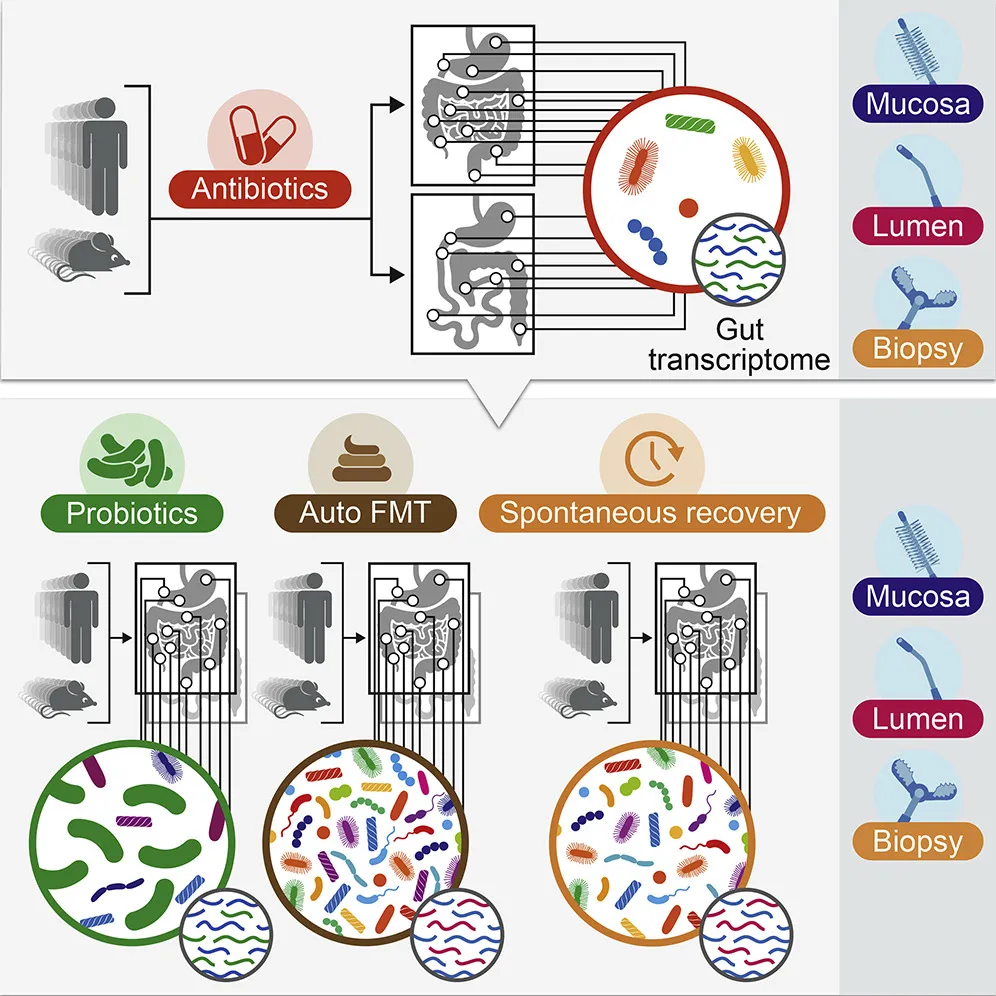
Probiotics are commonly used to help jumpstart the gut biome after a course of antibiotics, which can kill off beneficial bacteria. However scientific literature on the practice is mixed, and the FDA has not yet approved a single probiotic prep for medical applications.
The team gave 21 volunteers a mix of the antibiotics ciprofloxacin and metronidazole at standard dosages for a period of seven days, simulating the kind of treatment often used clinically for GI ailments from Crohn’s disease to diverticulitis. Patients were then separated into three groups. One group simply let their own microbiomes recover naturally, the second took generic probiotics, and the third was given a healthy dose of their own bacterial biome, which had been collected before the antibiotics use and re-administered via an autologous fecal microbiome transplant (aFMT).
The last group saw a full reversal of antibiotics effect. Reseeded with their own fecal material, the patients’ microbiomes returned to normal in just a few days. (Elinav’s group is pursuing a patent related to aFMT work.)
But those taking probiotics had a very different reaction. Generic probiotics did well at colonizing the gut, which makes sense since the indigenous microbiome was at least partially wiped out by antibiotics, but the probiotics significantly prohibited the natural biome from recovering and returning to its natural state. Even six months after the treatment, these patients’ natural biomes had not fully recovered, suggesting off-the-shelf replacement bacteria aren’t a great substitute for the wider diversity of natural microbiome.
“We’re talking about an entire rainforest in the gut that’s being affected in different ways by different antibiotics, and you can’t just patch that up by giving a probiotic,” Allen-Vercoe says. “Because let’s face it, a probiotic has maybe seven or eight strains. There’s a lot in the literature about some of these bacteria being beneficial, and it’s interesting, but they are really some of the few microbes in the gut that are fairly straightforward to culture. And I think that drives the probiotic industry more than it would like to admit.”
Ciorba adds that while the results don’t show any direct harm to patients recovering from antibiotics, they are certainly food for thought. “If we think that reconstitution to a normal and diverse microbiota is good, then potentially this predicts what could be a harm in a bigger population-based setting,” he says. “It highlights for patients and physicians that there may be situations where prolonged probiotic use may not be beneficial if diversity is the end point that we’re looking for.”
Jonathan Eisen, of the UC Davis Genome Center, notes that the human microbiome, like that found in the gut, is important for all types of health and disease states. Diversity does seem to be a key to its successes, but at the same time it’s a challenge for scientists aiming to aid its functions.
“It is deeply complex and complicated and is affected by a diversity of factors, including diet, immune status, behavior, genetics, interactions with other people and animals, the built environment, random forces, location, the weather, cleaning practices and much more,” Eisen said in an email. “Therefore, it is generally important for everyone to realize that we are unlikely to find simple rules (e.g., take this probiotic for this health issue) that work well across a large diversity of people in a wide range of conditions.”