Some Salamanders Can Regrow Lost Body Parts. Could Humans One Day Do the Same?
In recent decades, the idea of human regeneration has evolved from an ‘if’ to a ‘when’
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/a8/b6/a8b6bd50-be66-4fa5-b4de-86e4ff7058b5/42-33054253edit.jpg)
As amphibians go, axolotls are pretty cute. These salamanders sport a Mona Lisa half-smile and red, frilly gills that make them look dressed up for a party. You might not want them at your soiree, though: They’re also cannibals. While rare now in the wild, axolotls used to hatch en masse, and it was a salamander-eat-salamander world. In such a harsh nursery, they evolved — or maybe kept — the ability to regrow severed limbs.
“Their regenerative powers are just incredible,” says Joshua Currie, a biologist at the Lunenfeld-Tanenbaum Research Institute in Toronto who’s been studying salamander regeneration since 2011. If an axolotl loses a limb, the appendage will grow back, at just the right size and orientation. Within weeks, the seam between old and new disappears completely.
And it’s not just legs: Axolotls can regenerate ovary and lung tissue, even parts of the brain and spinal cord.
The salamander’s exceptional comeback from injury has been known for more than a century, and scientists have unraveled some of its secrets. It seals the amputation site with a special type of skin called wound epithelium, then builds a bit of tissue called the blastema, from which sprouts the new body part. But until recently, the fine details of the cells and molecules needed to create a leg from scratch have remained elusive.
With the recent sequencing and assembly of the axolotl’s giant genome, though, and the development of techniques to modify the creature’s genes in the lab, regeneration researchers are now poised to discover those details. In so doing, they’ll likely identify salamander tricks that could be useful in human medicine.
Already, studies are illuminating the cells involved, and defining the chemical ingredients needed. Perhaps, several decades from now, people, too, might regrow organs or limbs. In the nearer future, the findings suggest possible treatments for ways to promote wound-healing and treat blindness.
The idea of human regeneration has evolved from an “if” to a “when” in recent decades, says David Gardiner, a developmental biologist at the University of California, Irvine. “Everybody now is assuming that it’s just a matter of time,” he says. But, of course, there’s still much to do.
Rainbow regeneration
In a working limb, cells and tissues are like the instruments in an orchestra: Each contributes actions, like musical notes, to create a symphony. Amputation results in cacophony, but salamanders can rap the conductor’s baton and reset the remaining tissue back to order — and all the way back to the symphony’s first movement, when they first grew a limb in the embryo.
The basic steps are known: When a limb is removed, be it by hungry sibling or curious experimenter, within minutes the axolotl’s blood will clot. Within hours, skin cells divide and crawl to cover the wound with a wound epidermis.
Next, cells from nearby tissues migrate to the amputation site, forming a blob of living matter. This blob, the blastema, is “where all the magic happens,” said Jessica Whited, a regenerative biologist at Harvard University, in a presentation in California last year. It forms a structure much like the developing embryo’s limb bud, from which limbs grow.
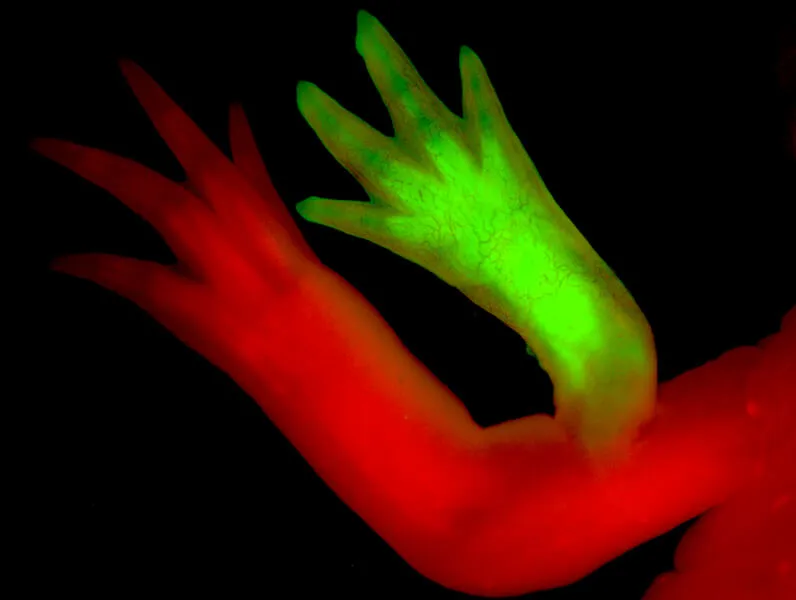
This movie shows immune cells, labeled to glow green, moving within a regenerating axolotl fingertip. Scientists know that immune cells such as macrophages are essential for regeneration: When they are removed, the process is blocked. (Credit: Josh Currie)
Finally, cells in the blastema turn into all the tissues needed for the new limb and settle down in the right pattern, forming a tiny but perfect limb. This limb then grows to full size. When all is done, “you can’t even tell where the amputation occurred in the first place,” Whited tells Knowable Magazine.
Scientists know many of the molecular instruments, and some of the notes, involved in this regeneration symphony. But it’s taken a great deal of work.
As Currie started as a new postdoc with Elly Tanaka, a developmental biologist at the Research Institute of Molecular Pathology in Vienna, he recalls wondering, “Where do the cells for regeneration come from?” Consider cartilage. Does it arise from the same cells as it does in the developing embryo, called chondrocytes, that are left over in the limb stump? Or does it come from some other source?
To learn more, Currie figured out a way to watch individual cells under the microscope right as regeneration took place. First, he used a genetic trick to randomly tag the cells he was studying in a salamander with a rainbow of colors. Then, to keep things simple, he sliced off just a fingertip from his subjects. Next, he searched for cells that stuck out — say, an orange cell that ended up surrounded by a sea of other cells colored green, yellow and so on. He tracked those standout cells, along with their color-matched descendants, over the weeks of limb regeneration. His observations, reported in the journal Developmental Cell in 2016, illuminated several secrets to the regeneration process.
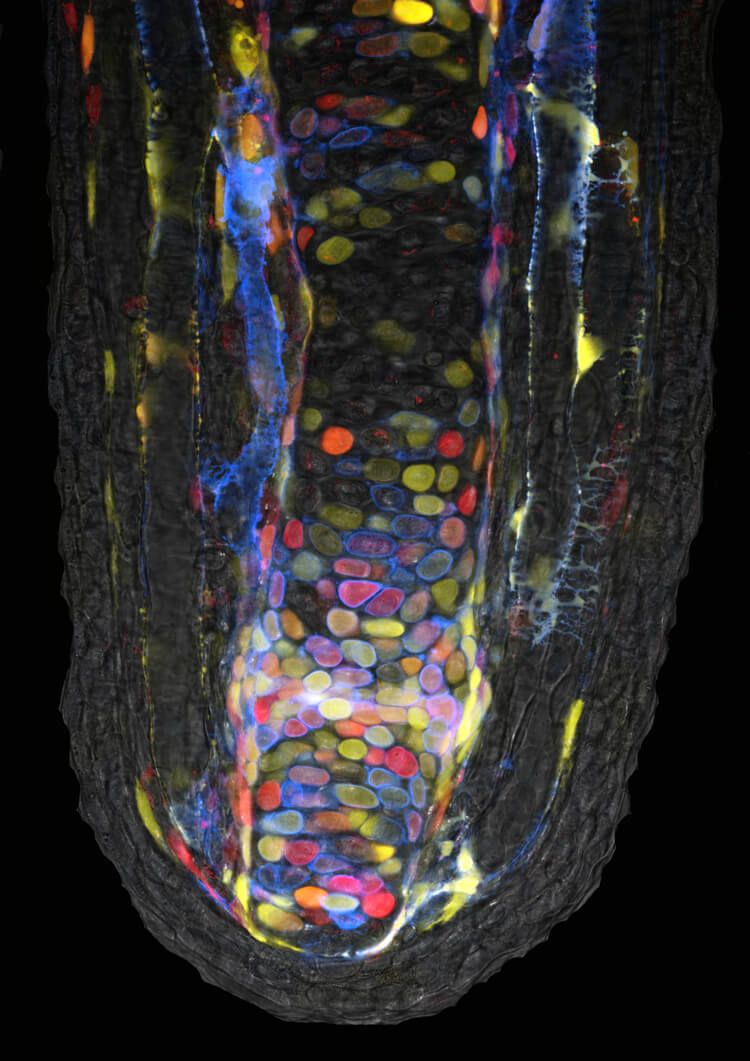
For one thing, cell travel is key. “Cells are really extricating themselves from where they are and crawling to the amputation plane to form this blastema,” Currie says. The distance cells will journey depends on the size of the injury. To make a new fingertip, the salamanders drew on cells within about 0.2 millimeters of the injury. But in other experiments where the salamanders had to replace a wrist and hand, cells came from as far as half a millimeter away.
More strikingly, Currie discovered that contributions to the blastema were not what he’d initially expected, and varied from tissue to tissue. “There were a lot of surprises,” he says.
Chondrocytes, so important for making cartilage in embryos, didn’t migrate to the blastema (earlier in 2016, Gardiner and colleagues reported similar findings). And certain cells entering the blastema — pericytes, cells that encircle blood vessels — were able to make more of themselves, but nothing else.
The real virtuosos in regeneration were cells in skin called fibroblasts and periskeletal cells, which normally surround bone. They seemed to rewind their development so they could form all kinds of tissues in the new fingertip, morphing into new chondrocytes and other cell types, too.
To Currie’s surprise, these source cells didn’t arrive all at once. Those first on the scene became chondrocytes. Latecomers turned into the soft connective tissues that surround the skeleton.
How do the cells do it? Currie, Tanaka and collaborators looked at connective tissues further, examining the genes turned on and off by individual cells in a regenerating limb. In a 2018 Science paper, the team reported that cells reorganized their gene activation profile to one almost identical, Tanaka says, to those in the limb bud of a developing embryo.
Muscle, meanwhile, has its own variation on the regeneration theme. Mature muscle, in both salamanders and people, contains stem cells called satellite cells. These create new cells as muscles grow or require repair. In a 2017 study in PNAS, Tanaka and colleagues showed (by tracking satellite cells that were made to glow red) that most, if not all, of muscle in new limbs comes from satellite cells.
Recipe for regeneration
If Currie and Tanaka are investigating the instruments of the regeneration symphony, Catherine McCusker is decoding the melody they play, in the form of chemicals that push the process along. A regenerative biologist at the University of Massachusetts Boston, she recently published a recipe of sorts for creating an axolotl limb from a wound site. By replacing two of three key requirements with a chemical cocktail, McCusker and her colleagues could force salamanders to grow a new arm from a small wound on the side of a limb, giving them an extra arm.
The first requirement for limb regeneration is the presence of a wound, and formation of wound epithelium. But a second, scientists knew, was a nerve that can grow into the injured area. Either the nerve itself, or cells that it talks to, manufacture chemicals needed to make connective tissue become immature again and form a blastema. In their 2019 study in Developmental Biology, McCusker and colleagues — guided by earlier work by a Japanese team — used two growth factors, called BMP and FGF, to fulfill that step in salamanders lacking a nerve in the right place.
The third requirement was for fibroblasts from opposite sides of a wound to find and touch each other. In a hand amputation, for example, cells from the left and right sides of the wrist might meet to correctly pattern and orient the new hand. McCuscker’s chemical replacement for this requirement was retinoic acid, which the body makes from vitamin A. The chemical plays a role in setting up patterning in embryos and has long been known to pattern tissues during regeneration.
In their experiment, McCusker’s team removed a small square of skin from the upper arm of 38 salamanders. Two days later, once the skin had healed over, the researchers made a tiny slit in the skin and slipped in a gelatin bead soaked in FGF and BMP. Thanks to that cocktail, in 25 animals the tissue created a blastema — no nerve necessary.
About a week later, the group injected the animals with retinoic acid. In concert with other signals coming from the surrounding tissue, it acted as a pattern generator, and seven of the axolotls sprouted new arms out of the wound site.
The recipe is far from perfected: Some salamanders grew one new arm, some grew two, and some grew three, all out of the same wound spot. McCusker suspects that the gelatin bead got in the way of cells that control the limb’s pattern. The key actions produced by the initial injury and wound epithelium also remain mysterious.
“It’s interesting that you can overcome some of these blocks with relatively few growth factors,” comments Randal Voss, a biologist at the University of Kentucky in Lexington. “We still don’t completely know what happens in the very first moments.”
Once upon a time
If we did know those early steps, humans might be able to create the regeneration symphony. People already possess many of the cellular instruments, capable of playing the notes. “We use essentially the same genes, in different ways,” says Ken Poss, a regeneration biologist at the Duke University Medical Center in Durham who described new advances in regeneration, thanks to genetic tools, in the 2017 Annual Review of Genetics.
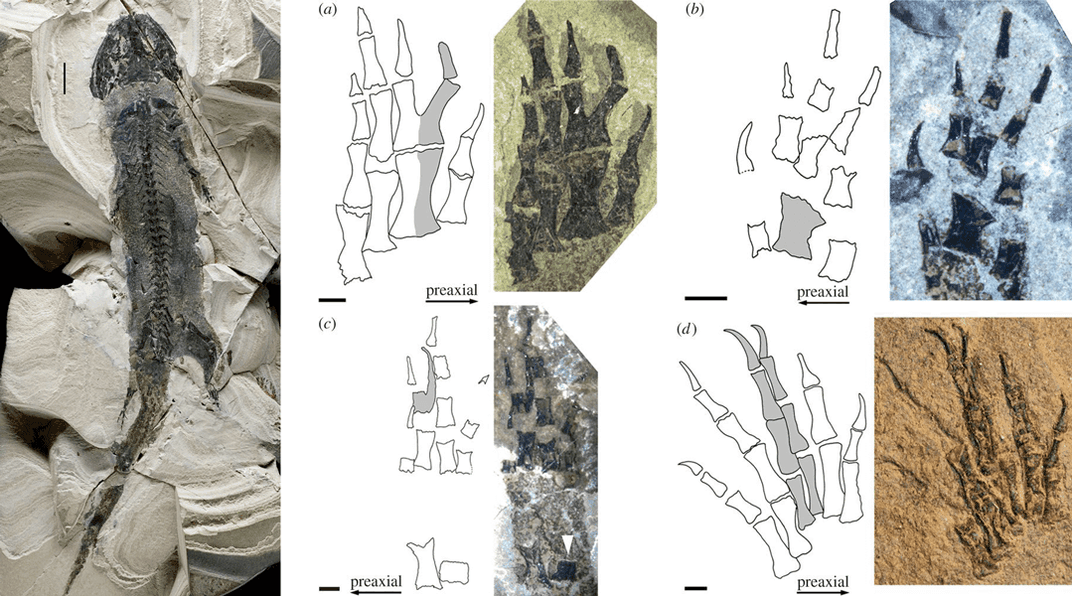
Regeneration may have been an ability we lost, rather than something salamanders gained. Way back in our evolutionary past, the common ancestors of people and salamanders could have been regenerators, since at least one distant relative of modern-day salamanders could do it. Paleontologists have discovered fossils of 300-million-year-old amphibians with limb deformities typically created by imperfect regeneration. Other members of the animal kingdom, such as certain worms, fish and starfish, can also regenerate — but it’s not clear if they use the same symphony score, Whited says.
Somewhere in their genomes, “all animals have the ability,” says James Monaghan, a regeneration biologist at Northeastern University in Boston. After all, he points out, all animals grow body parts as embryos. And in fact, people aren’t entirely inept at regeneration. We can regrow fingertips, muscle, liver tissue and, to a certain extent, skin.
But for larger structures like limbs, our regeneration music falls apart. Human bodies take days to form skin over an injury, and without the crucial wound epithelium, our hopes for regeneration are dashed before it even starts. Instead, we scab and scar.
“It’s pretty far off in the future that we would be able to grow an entire limb,” says McCusker. “I hope I’m wrong, but that’s my feeling.”
She thinks that other medical applications could come much sooner, though — such as ways to help burn victims. When surgeons perform skin grafts, they frequently transfer the top layers of skin, or use lab-grown skin tissue. But it’s often an imperfect replacement for what was lost.
That’s because skin varies across the body; just compare the skin on your palm to that on your calf or armpit. The tissues that help skin to match its body position, giving it features like sweat glands and hair as appropriate, lie deeper than many grafts. The replacement skin, then, might not be just like the old skin. But if scientists could create skin with better positional information, they could make the transferred skin a better fit for its new location.
Monaghan, for his part, is thinking about regenerating retinas for people who have macular degeneration or eye trauma. Axolotls can regrow their retinas (though, surprisingly, their ability to regenerate the lens is limited to hatchlings). He is working with Northeastern University chemical engineer Rebecca Carrier, who’s been developing materials for use in transplantations. Her collaborators are testing transplants in pigs and people, but find most of the transplanted cells are dying. Perhaps some additional material could create a pro-regeneration environment, and perhaps axolotls could suggest some ingredients.
Carrier and Monaghan experimented with the transplanted pig cells in lab dishes, and found they were more likely to survive and develop into retinal cells if grown together with axolotl retinas. The special ingredient seems to be a distinct set of chemicals that exist on axolotl, but not pig, retinas. Carrier hopes to use this information to create a chemical cocktail to help transplants succeed. Even partially restoring vision would be beneficial, Monaghan notes.
Thanks to genetic sequencing and modern molecular biology, researchers can continue to unlock the many remaining mysteries of regeneration: How does the wound epithelium create a regeneration-promoting environment? What determines which cells migrate into a blastema, and which stay put? How does the salamander manage to grow a new limb of exactly the right size, no larger, no smaller? These secrets and more remain hidden behind that Mona Lisa smile — at least for now.


