NATIONAL MUSEUM OF NATURAL HISTORY
The Strangely Scientific Endeavor of Making Ice Cream
Ice cream’s texture is the result of the same processes that govern concepts like forest recovery, rock formation and sub-zero survival in animals.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/blogging/featured/Hand_holding_a_sugar_cone_full_of_light_green_ice_cream.jpg)
When you think about ice cream, you might marvel at the plethora of available flavors. Or relish in the refreshment a scoop brings on a hot summer day. But there’s more to ice cream than meets the mouth. Its unique and delectable texture is the result of the same physical and chemical processes that govern concepts like forest recovery, rock formation and sub-zero survival in animals.
Here are five cool connections to ponder while you enjoy your next cone, cup or pint.
Rock-y road
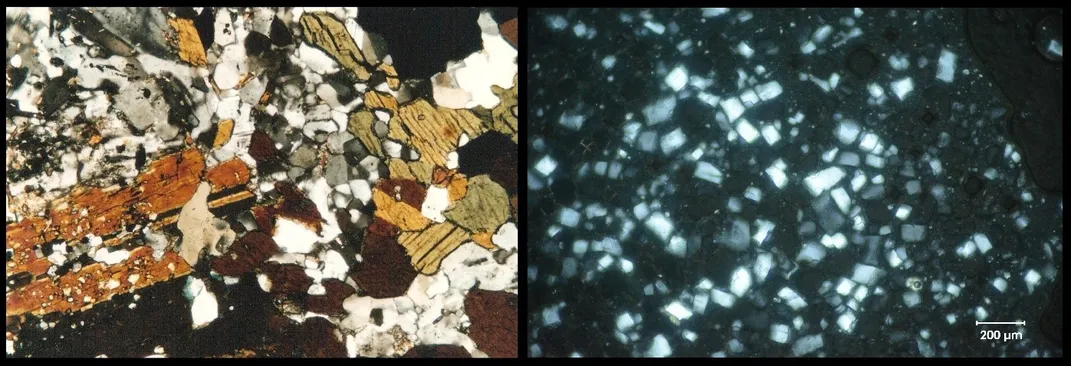
One of the main ingredients in ice cream is water, mostly in the form of microscopic ice crystals. The size of those crystals plays a big role in ice cream quality. Large crystals create a grainy texture, whereas smaller crystals — as little as blood cells — make it velvety smooth. So how do ice cream makers keep the little ice nuggets from growing larger than a dozen micrometers?
One way is knowing that ice is just as much a mineral as quartz or graphite. And in some ways, it behaves like them too. “Looking at ice cream under a microscope is not that different from looking at a piece of granite or other rock that’s cooled from magma in the Earth,” says Jeffrey Post, Curator-in-Charge of Gems and Minerals at the Smithsonian’s National Museum of Natural History.
When minerals solidify from liquid lava or magma, “their crystals provide certain clues about the conditions under which they formed,” said Post. For example, thick, gooey magma cooling slowly deep in the earth, allows crystals to grow. Thin and runny lava at the surface cools and hardens much faster, producing rocks with smaller crystals.
Sweeteners and stabilizers thicken ice cream to slow crystal growth, but another way keep the crystals small is to speed up the freezing process. Adding liquid nitrogen, which freezes the ice cream on contact, has increased in popularity over the years. Its extremely cold temperature creates smooth ice cream in only a few minutes.
Mint chip or wood chips?

Another way to keep crystals from growing is to chop them down as soon as they start to form in the mixing container. In the first stage of ice cream creation, called dynamic freezing, the mixer constantly scrapes newly formed crystals from the walls of the bucket, churning them into the middle of the mixture. This not only stops crystals from growing thick on the inner walls, but also creates more nuclei, or crystal origin points, for liquid water molecules to freeze onto. As Post explains, “all of those smaller crystals are now competing with each other for the remaining water molecules, so none of them can grow really large.”
This process closely resembles the effect that clear-cutting, hurricanes or intense wildfires can have on forests. When a stand of trees is chopped, burned or blown down, densely packed saplings grow in its place at a uniform pace. It can take several decades before the weaker ones die off and make room for the stronger individuals. In the meantime, the regenerating “second growth” forest is stunted as the overcrowded trees compete for limited resources. For forests, slow growth and varied sizes generally yield a healthier ecosystem. But for ice cream, clear cuts and competition are key to a creamy texture.
Chocolate chip antifreeze

Once ice cream is made, it’s best to eat it fresh and all in one go. But if filling up on frozen desserts isn’t an option, you must store them, sometimes for weeks or months. During this time, the temperature of the ice cream might fluctuate as freezer doors open and close. If it melts even a little, the ice will recrystallize, growing bigger crystals over time. The result: an icy, crunchy texture that just isn’t worth six dollars per pint.
By slowing down the movement of liquid water molecules within the ice cream mixture, thickeners and stabilizers keep things running smoothly for long periods of time. But when that’s not enough, ice cream makers have looked to cold-adapted wildlife for help.
Several species of frogs, insects and plants evolved antifreeze proteins in their tissues to help them survive in frigid conditions. These proteins surround and bind to ice crystals as soon as they form in the body. By blocking liquid water molecules from bonding with the budding crystals, the antifreeze allows organisms to avoid cell damage and even death.
Antifreeze proteins originally discovered in cold-water fish and then synthesized in the lab via genetically modified yeasts have been applied to ice creams worldwide to inhibit ice recrystallization.
Physics and cream

Oil and water repel each other. So why doesn’t ice cream — a mixture of mostly ice and milk fats — separate into two layers? The answer can be found in its microscopic structure.
If you shake a bottle with oil and vinegar in it, the oil breaks up into small, spherical droplets. If left undisturbed, the droplets will eventually coalesce back into a layer at the surface. But the two liquids can appear to become one if they are vigorously shaken or blended at high speed. They become an emulsion — an even dispersion of two unmixable liquids.
Most unmixable mixtures are thermodynamically unstable, meaning they will eventually revert to a simpler, more organized structure with one liquid sitting on top of the other. But stable emulsions are different. No matter how long you wait, the fats won’t rise to the top. Coconut water and homogenized milk are two familiar examples of stable emulsions.
These oil-in-water substances stay evenly dispersed partly because they contain natural emulsifying proteins which work in a similar way as antifreeze proteins. Instead of binding to the ice, emulsifiers latch onto the fat droplets and lower the tension between the two liquids, preventing the fat from aggregating and forming its own layer.
In ice cream, milk proteins keep things relatively stable. But extra emulsifiers like lecithin or casein are often needed to help another major ingredient — air — stay in the mix. Tiny air bubbles make ice cream more scoopable and help soft serve keep its shape, but only if they also remain small and evenly distributed amongst the fat and ice.
Cookies and Crystalline

Naturally occurring ice comes in many different shapes and sizes, from hollow columns and needles to platelets and bullet-shaped rosettes. Whichever shape an ice crystal takes, it largely depends on the humidity and temperature surrounding the crystal during formation. Higher humidity produces bigger, more elaborate snowflakes.
Most of these crystal shapes need time, space and moist air to grow or branch out, and a churning ice cream machine furnishes no such amenities. Instead, ice cream crystals more closely resemble the simple prisms or platelets that form in very cold, dry conditions. The constant movement by the mixer also wears the crystals down like the ocean wears down sand, resulting in microscopic, irregular grains.
While the ice crystals in your sundae may look like nothing more than tiny pebbles, they do make great food for thought. “All processes on Earth are controlled by the same physics and chemistry, whether it’s ice cream, rock formation inside the Earth or weather up in the sky,” said Post. “If we understand the physics and chemistry, then we can understand our world — and we can create a better ice cream.” And who doesn’t want better ice cream?
Related stories:
Why Scientists Find Snowflakes Cool
How Seven of Nature’s Coolest Species Weather the Cold
How to Identify Rocks and Other Questions From Our Readers

