The Ozone Problem is Back – And Worse Than Ever
James Anderson, the winner of a Smithsonian American Ingenuity Award, has discovered the alarming link between climate change and ozone loss
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Ingenuity-Awards-Jim-Anderson-631.jpg)
“Bull!” said Kerry Emanuel, an atmospheric scientist at MIT.
Jim Anderson of Harvard University was showing him some weird data he had collected. Since 2001, Anderson and his team had been studying powerful thunderstorms by packing instruments into repurposed spy planes and B-57 bombers, among the only planes capable of flying into the storms “without having their wings ripped off,” Anderson said. To his puzzlement, the instruments detected surprisingly high concentrations of water molecules in the stratosphere, the usually drier-than-dust uppermost layer of the atmosphere. They found the water over thunderstorms above Florida, and they found it over thunderstorms in Oklahoma—water as out of place as a dolphin in the Sahara.
While water in the stratosphere might seem innocuous, the finding made Anderson “profoundly worried,” he recalls. From the decades he had spent studying the depletion of the earth’s ozone layer—the thin gauze of molecules in the stratosphere that blocks most incoming ultraviolet radiation—Anderson knew that water could, through a series of chemical reactions, destroy ozone.
It was when he told Emanuel that violent thunderstorms seemed to be heaving water high into the atmosphere that his MIT colleague expressed his skepticism. A quick back-of-the-envelope calculation showed “you’d need an updraft of 100 miles an hour” to do that, Emanuel said. Impossible.
Anderson persisted, and by early 2012 he had demonstrated the connection. Scrutinizing data from the high-altitude flights, he showed that summer thunderstorms were indeed injecting water molecules into the stratosphere. There, sulfate aerosols (from industrial pollutants as well as volcanoes) attract the water molecules like a sponge and, plumped up, provide a bed for chemical reactions that destroy ozone. The destruction can persist for days or weeks. Oh, and one more thing: The violent storms that inject water vapor into the stratosphere might be getting more powerful and more frequent under the influence of global warming.
Anderson had made a revolutionary connection between climate change and ozone loss. For three decades, scientists have shouted themselves hoarse insisting the two planetary threats were separate and unrelated. “What Anderson did is piece together all of the complicated parts—how you can inject water in higher and higher amounts into the upper atmosphere and how that causes ozone destruction—and come up with this alarming possibility,” says atmospheric scientist Ralph Cicerone, president of the National Academy of Sciences, who has done pioneering work on the ozone layer. “He’s identified a really important mechanism.”
And if Anderson is right and the ozone layer is under renewed attack, then all of the potential consequences of that threat are back like a bad dream from the 1980s: more ultraviolet light reaching the ground; more cases of skin cancer and cataracts; damage to plankton and other organisms that support ocean life; and withered crops that could lead to skyrocketing food prices.
***
Anderson, courtly and white-haired at 68, is writing in longhand at his desk in Harvard’s Mallinckrodt Laboratory early on a sunny autumn morning. The surrounding offices are still dark and empty; Anderson has been at it for over an hour, he says.
But scholarly research isn’t his only passion. He’s also shown unusual devotion to teaching undergrads, lacing an introductory physical sciences class with pragmatic case studies, such as having students calculate their personal energy use. “When I started, I was teaching freshman chemistry the old way, where the idea was to flunk 90 percent of the students,” says Anderson. “But that wastes a huge amount of creative talent and drives students away from science, never to return.”
So Anderson revamped the course, doing his best to liven it up. “Every day he made something explode or set something on fire,” says Adam Cohen, an associate professor who teaches the course now. Anderson has since poured his teaching philosophy into a chemistry textbook he has been writing for years. It’s almost ready for publication, and he proudly shows off the cover he designed adorned with a zippy red Tesla, the high-end electric car. He has one on order. (Read more about the Tesla and its creator on p. 72.)
Anderson’s love of research took root early, in the machine shop that his father, chairman of the physics department at Washington State University in Pullman, built in the family’s basement. It was there that Anderson, born in 1944, built his first model plane, at age 6, and where by seventh grade he was constructing boats.
During summers with his grandparents at Lake Pend Oreille in Idaho—a retreat where he and his wife still vacation—he repaired outboard motors and built treehouses, forts, rafts and radios (“there were none except when we built them”). After majoring in physics at the University of Washington, Anderson found his life’s calling during his graduate student years at the University of Colorado.
At its Laboratory for Atmospheric and Space Physics in Boulder, he devised a way to measure very low concentrations of free radicals—clusters of atoms that carry an electric charge—in the stratosphere. “Free radicals are the Lord God of all chemical transformations,” Anderson says with the enthusiasm of a little kid for things that go boom: They serve as catalysts for everything from rusting to smog formation. The measuring device he came up with could detect concentrations of free radicals as low as one part in a trillion, equivalent to a couple of grains of salt in an Olympic-size pool, and was carried aloft by a rocket.
Figuring out how to shoot scientific instruments into space came in handy. In the 1970s and ’80s, several teams of scientists were warning that technologies as different as deodorant cans and the space shuttle were spewing all sorts of chemicals into the atmosphere with possibly disastrous effects for the ozone layer. Arguably the most threatening were industrial gases called chlorofluorocarbons (CFCs) from aerosol cans, air conditioners and refrigerators. Were those products injecting massive amounts of CFCs into the stratosphere? By 1979, using instruments carried into the stratosphere on balloons lofted from the National Scientific Balloon Facility in Palestine, Texas, Anderson and his team detected the telltale signature of CFCs. They really were reaching the stratosphere in measurable quantities.
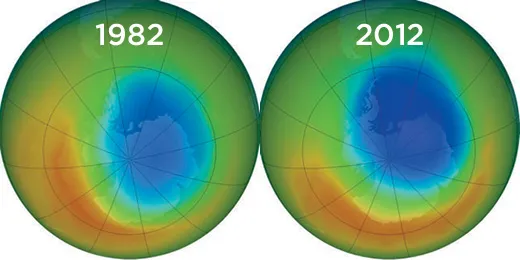
But were they causing harm? Circumstantial evidence was pouring in, none more stunning than a discovery announced by scientists with the British Antarctic Survey in 1985: A massive hole in the ozone layer had opened up over the South Pole. The ozone layer there was 60 to 70 percent thinner than usual. A 10 percent drop in ozone thickness allows 10 percent more UV sunlight to reach the earth’s surface; 10 percent more UV will lead to a 20 to 30 percent increase in the most common forms of skin cancer, the Environmental Protection Agency calculates. If that much ozone depletion occurred over inhabited regions rather than the South Pole, cancer rates could soar.
And yet ever-cautious scientists were still not ready to declare CFCs the culprits. Anderson ran the definitive experiment. In 1987, instruments he and his team built flew aboard NASA’s ER-2 aircraft—the civilian version of the U-2 spy plane—in the Airborne Antarctic Ozone Experiment.
Scientists do not keep aircraft, or even balloons, on standby, of course. Instead, “NASA announces a field mission with a specific goal in mind and asks experimenters to take part,” says Lenny Solomon, who managed Anderson’s lab and logistics from 1978 until his “retirement” in 2009. (Less than a year later Anderson asked Solomon to come back one day a week.) NASA and the balloon facility also “send out yearly questionnaires to investigators asking if they’d like some flight time and for what reasons,” Solomon says—an offer Anderson rarely passed up.
From August to September, the ER-2s took off into the lower stratosphere from Punta Arenas, Chile, whose military was on alert over tensions with Argentina. “Night raids were being launched out of the next hangar” beside their own NASA craft, recalls Anderson. “We had 18-year-olds guarding us with AK-47s.”
Anderson finally got his free radical. His instruments achieved the first detection of chlorine monoxide, ClO, in the stratosphere. The only source of ClO is ozone destruction by chlorine. Moreover, Anderson found that the higher the concentration of ClO the lower the concentration of ozone. “That anti-correlation between ClO and ozone was a dramatic clue to what was happening,” Anderson says. His lab work had shown how quickly a given concentration of ClO makes ozone disappear. The numbers matched: The ClO they detected in the stratosphere was exactly the right concentration to explain the measured ozone loss. It was the smoking gun proving that CFCs were chomping away at the ozone layer like so many high-altitude Pac-Men.
It was Anderson’s most important contribution to science to date. And it was the final piece of the puzzle needed to move public policy, culminating in the 1987 Montreal Protocol, now signed by 197 countries that agreed to phase out CFCs.
In 2005, the United Nations lauded Anderson for “his elegant measurements and brilliant analysis of ClO radical concentrations over Antarctica,” demonstrating how CFCs are “responsible for the observed massive springtime ozone depletion.”
The rest of the world may have thought the ozone problem had been solved, but Anderson wasn’t so sure. He persisted in his high-altitude research forays. ER-2 flights from Bangor, Maine, in 1992, found “extremely high ClO over the United States,” he recalls. In 2000, flights from Sweden showed that “the arctic was beginning to emulate” the “massive ozone loss” over Antarctica, as he put it. (The Sweden mission was slightly delayed when a Russian general, who was scheduled to fly in a DC-8 chase plane with Anderson as the spy plane flew over Russia, vanished briefly. Anderson thought he had been going to the head, but the general was taking forever. It turned out he was conferring by phone with officials in the Kremlin, telling them one last time that the U-2 they’d soon notice in Russia’s skies was doing science, not espionage, and to please not shoot it down.)
Those discoveries should have served as a wake-up call that, for all the good the Montreal Protocol did, ozone loss was not a thing of the past. “But NASA [which had funded much of Anderson’s work] said we’re declaring victory against ozone loss and going after climate change by studying clouds,” he says. Among the many unknowns about how climate will change in a world warmed by a blanket of greenhouse gases—mostly carbon dioxide from burning fossil fuels—is whether clouds will slow or accelerate global warming.
Anderson decided to tackle one piece of that puzzle: the formation of cirrus clouds. Clouds, of course, are made of water vapor. On summertime flights to measure water vapor starting in 2001, Anderson’s team kept getting “deadly boring” results, the same 4.5 to 5 parts per million of water in the stratosphere. In 2005 and 2007, however, flights over Florida and then Oklahoma found “to our shock and surprise,” Anderson says, that thunderstorms were injecting water molecules as high as 12 miles into the stratosphere, reaching the ozone layer. It wasn’t a rare event, either: About half the flights found the high-altitude water. As Anderson and his colleagues wrote with the usual academic understatement in Science last summer, “What proved surprising is the remarkable altitude to which large concentrations of water vapor are observed to penetrate.”
“I went to NASA and said we have a big problem here,” says Anderson. Go away, the agency told him; we’ve moved on, now that the world had solved the ozone problem by phasing out CFC production.
Anderson persisted (again) and began writing more and more insistent letters up the NASA chain of command. He finally got a sympathetic hearing from Ken Jucks, manager for the agency’s Upper Atmosphere Research Program. Together, they wrested enough financial support for Anderson to keep his team together and analyze what the raw data from the flights were trying to tell him.
What happens is that the strong thunderstorms—those about 30 miles across—create powerful updrafts, essentially gaseous elevators that carry warm, humid air miles into the atmosphere. Usually, the gaseous elevator stops short of the stratosphere. But if a storm is strong enough, the updraft can blast through the boundary between the lower atmosphere and the stratosphere, reaching the latter and spreading 60 miles or more in all directions and remaining there for days. The concentration of water in the stratosphere more than triples.
The more water, the more ozone loss, through a sequence that begins with the fact that as the air rises, it cools. (To test this, put your hand against the window of an airplane the next time you fly.) The water vapor condenses out as liquid water, much as the steam from your shower turns liquid when it hits a cold bathroom mirror. Condensation releases heat. That raises the temperature of the surrounding air, which contains CFCs left over from the days before they were banned.
The heat alters CFC molecules in such a way as to make them more reactive; specifically, sunlight breaks apart the chlorine molecules in CFCs, producing ClO, the same free radical whose detection by Anderson’s team provided the final proof that CFCs destroy ozone over Antarctica. That free radical, Anderson’s latest work showed, is also—thanks to powerful thunderstorms—chomping its way through the ozone layer over the U.S.
As a result, ozone is depleted 100 times faster in an area affected by thunderstorms than in an area that is not. About 13 to 21 percent of the ozone is destroyed after four days, with losses of 4 to 6 percent over the next few days. All told, 25 to 30 percent of the ozone over a 60- by 60-mile area could be destroyed, with the effect persisting for weeks. Sunlight eventually replenishes the molecule, converting ordinary oxygen into it; one big remaining question is whether ozone destruction or replenishment will come out ahead. The region the storm-tossed water reaches, 9 to 12 miles up, contains about 20 percent of the ozone column in the summer over the U.S.
“The system reacts much more quickly than we expected,” says Anderson. “We don’t know how long that lasts, but it may be many days or weeks.” If the intensity and frequency of powerful summertime thunderstorms increased as a result of climate change, he and his colleagues wrote, then “decreases in ozone and associated increases in UV dosage would also be irreversible”—at least until there are no more manmade chlorine or other ozone-eating chemicals in the atmosphere.
In 80 years or so, CFCs from the air conditioner in your 1965 Mustang, the spray cans that were part of your morning grooming and every other source will have finally dissipated, eliminating the threat to ozone. Accordingly, that means we’ll have to hang on for another eight decades with possibly more people dying from skin cancer and more crops wilting under the intense UV rays.
To be sure, the idea of ozone-killing storms is not a slam-dunk at this point. The weakest link in the chain of evidence is whether climate change is indeed bringing more powerful and more frequent thunderstorms. “We haven’t a clue whether that’s happening,” says MIT’s Emanuel, “but Jim’s work shows that we better pay attention to the connection” between climate change and thunderstorms.
Anderson acknowledges the uncertainty—“we can’t write down a precise equation between carbon dioxide and storms”—but is convinced the link is there, partly because rising levels of greenhouse gases have already been accompanied by weird rainfall patterns: Since the late 1950s, the percentage of rainfall coming in deluges has increased some 70 percent in the Northeast and 30 percent in the Midwest, for instance. Climate scientist James Hansen believes Anderson is right: “What we call ‘moist convection’ will penetrate higher into the atmosphere as the climate becomes warmer,” he says.
Anderson’s work brings the science of ozone loss full circle. Years before some scientists suspected that chlorine from CFCs attacked stratospheric ozone, others warned that supersonic aircraft such as the now-retired Concorde could deplete the ozone layer because its exhaust left water molecules in the stratosphere. Jim Anderson showed that something much more common—the thunderstorms that characterize American summers as reliably as watermelon and hot dogs—can provide the ozone-destroying water. “We thought we’d solved the problem of ozone depletion,” says Anderson, “but we haven’t. If anything, it could be made much worse than we thought by climate change.”