Living Cells Armed With Tiny Lasers May Help Fight Disease
The biological light sources may one day help researchers see deeper into the body’s microscopic workings
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/9a/64/9a6471ba-2f70-4815-b115-386d23ed980b/cell-lasers.jpg)
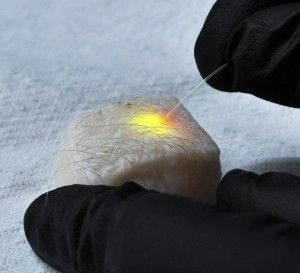
For all those cell biologists who had one simple request—cells equipped with miniature frickin' laser beams—a Harvard team has actually made it happen. The ability to direct tiny lasers on a fantastic voyage inside the body could enable a suite of medical applications, from delivering drugs to tracking tumor growth.
“We hope to use the cell as a biological machine, programmed by the DNA inside, that can deliver a laser to a target,” explains Seok-Hyun (Andy) Yun of Harvard Medical School.
Light is used to see inside cells with instruments like the single-cell endoscope, but its use has been limited to more accessible locations like the skin, because its light doesn't penetrate well into deeper tissue. Adding fluorescent dyes and proteins to cells can help scientists spot and examine them further inside the body. But those procedures produce a broad spectrum of emissions, which can make it difficult to pick out cell-specific data from among all the background emission produced by molecules in biological tissue.
Enter the microlaser, which may provide a far more precise and penetrating way to image, monitor and perhaps even aid living cells.
“We want to reinvent the laser for medical applications,” Yun says. “Instead of borrowing the lasers that have been invented by industry for various other reasons, we've made it with a biological material, and very small, so it can be implanted or injected into the body with few problems to do light-based applications where it's currently not practical to deliver light.”
A typical laser excites atoms so that they emit light at a particular wavelength, then bounces the light between a pair of mirrors to amplify the effect. One of the mirrors is partially transparent, allowing some light to escape in a narrow beam—that's the laser. The key to building a laser inside a cell is creating an optical microresonator—a miniature version of this setup that confines light so that it circulates inside a small sphere, where it's trapped by refraction at the sphere's surface.
Yun's team did this in two different ways. A soft version was made by injecting a small drop of oil or natural fat lipids mingled with a fluorescent dye into a cell. A hard version used fluorescent polystyrene beads instead. In each case, the entire cell was excited by a nanosecond pulse that produced light, which then became trapped inside the sphere.
“It's like when you're in an empty room and a certain voice frequency gets resonated,” Yun explains. “But if the room is squeezed, if the shape and size changes, the resonant frequency also gets altered. We do the same thing, in principle, with the optical frequency scale. Certain light gets resonated and, as it circulates the cavity, it gets amplified and eventually turns into the laser output.”
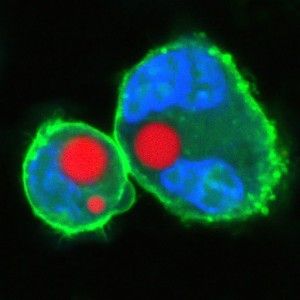
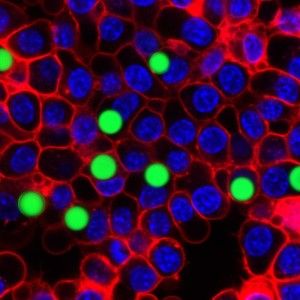
The extreme precision of that output is one thing that makes the tiny lasers so promising. The soft droplet versions shift shape ever so lightly when under stress, and that deformation makes a visible change in the laser's emission spectrum, so that even minute changes in the cell can be recorded in fine detail. Similarly the team can produce lasers of slightly different wavelengths by changing the size of the hard beads—enabling them to uniquely color code an individual cell and potentially label thousands of different cells within a single tissue, according to the research published this week in Nature Photonics.
Living cells are the ideal delivery mechanism for getting these microlasers where they can do the most good. For example, immune cells can be targeted to respond to specific problems, so they could deliver a laser to bind with a tumor or other disease location. Once in place, a finely tuned laser light could perform any number of applications.
“The spectral peaks of the laser are very sensitive to the local environment, and you can design the laser so it senses certain biomarkers and changes the output wavelength when they change even in tiny shifts,” Yun notes. This means the laser can deliver highly detailed information about cell surfaces, hormones and even the cell's protein production. The lasers could also be used to tag individual cells and thus paint a far more detailed picture of how a larger object, like a tumor, changes over time.
“You could see exactly where the individual cells go in the body, which ones metastasize earlier than others, and study the growth of shrinkage of a tumor at the individual cell level,” Yun says.
Perhaps most promising of all is the potential to not only monitor aspects of human health but to actively improve them, he adds: “These laser-equipped cells could also potentially be loaded with light-activated drugs and delivered to a specific location, where they might be used to kill a tumor, for example.”