How the Tree Frog Has Redefined Our View of Biology
The world’s most charismatic amphibian is upending the conventional wisdom about evolution
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Frog-that-Roared-red-eyed-tree-frog-631.jpg)
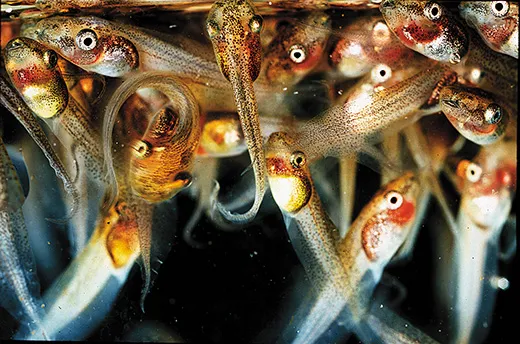
Karen Warkentin, wearing tall olive-green rubber boots, stands on the bank of a concrete-lined pond at the edge of the Panamanian rainforest. She pulls on a broad green leaf still attached to a branch and points out a shiny clutch of jellylike eggs. “These guys are hatchable,” she says.
Red-eyed tree frogs, Agalychnis callidryas, lay their eggs on foliage at the edge of ponds; when the tadpoles hatch, they fall into the water. Normally, an egg hatches six to seven days after it is laid. The ones that Warkentin is pointing to, judging from their size and shape, are about five days old, she says. Tiny bodies show through the clear gel-filled membrane. Under a microscope, the red hearts would just be visible.
She reaches down to wet her hand in the pond water. “They don’t really want to hatch,” she says, “but they can.” She pulls the leaf out over the water and gently runs a finger over the eggs.
Sproing! A tiny tadpole breaks out. It lands partway down the leaf, twitches and falls into the water. Another and another of its siblings follow. “It’s not something I get tired of watching,” Warkentin says.
With just a flick of her finger, Warkentin has demonstrated a phenomenon that is transforming biology. After decades of thinking of genes as a “blueprint”—the coded DNA strands dictate to our cells exactly what to do and when to do it—biologists are coming to terms with a confounding reality. Life, even an entity as seemingly simple as a frog egg, is flexible. It has options. At five days or so, red-eyed tree frog eggs, developing right on schedule, can suddenly take a different path if they detect vibrations from an attacking snake: They hatch early and try their luck in the pond below.
The egg’s surprising responsiveness epitomizes a revolutionary concept in biology called phenotypic plasticity, which is the flexibility an organism shows in translating its genes into physical features and actions. The phenotype is pretty much everything about an organism other than its genes (which scientists call the genotype). The concept of phenotypic plasticity serves as an antidote to simplistic cause-and-effect thinking about genes; it tries to explain how a gene or set of genes can give rise to multiple outcomes, depending partly on what the organism encounters in its environment. The study of evolution has so long centered on genes themselves that, Warkentin says, scientists have assumed that “individuals are different because they’re genetically different. But a lot of the variation out there comes from environmental effects.”
When a houseplant makes paler leaves in the sun and a water flea grows spines to protect against hungry fish, they’re showing phenotypic plasticity. Depending on the environment—whether there are snakes, hurricanes or food shortages to deal with—organisms can bring out different phenotypes. Nature or nurture? Well, both.
The realization has big implications for how scientists think about evolution. Phenotypic plasticity offers a solution to the crucial puzzle of how organisms adapt to environmental challenges, intentionally or not. And there is no more astonishing example of inborn flexibility than these frog eggs—blind masses of goo genetically programmed to develop and hatch like clockwork. Or so it seemed.
Red-eyed tree frog hatchlings were dodging hungry snakes a long time before Warkentin started studying the phenomenon 20 years ago. “People had not thought of eggs as having the possibility to show this kind of plasticity,” says Mike Ryan, her PhD adviser at the University of Texas in Austin. “It was very clear, as she was doing her PhD thesis, that this was a very, very rich field that she had sort of invented on her own.”
Karen Martin, a biologist at Pepperdine University, also studies hatching plasticity. “Hatching in response to some kind of threat has been a very important insight,” Martin says. “I think she was the first one to have a really good example of that.” She praises Warkentin’s sustained effort to learn big biology lessons from frog eggs: “I think a lot of people might have looked at this system and said, ‘Here’s a kind of a quirky thing that I could get some papers out of, and now I’ll move on and look at some other animal.’ She dedicated herself to understanding this system.”
Warkentin’s research “causes us to think more carefully about how organisms respond to challenges even very early in life,” says Eldredge Bermingham, an evolutionary biologist and director of the Smithsonian Tropical Research Institute (STRI, pronounced “str-eye”) in Gamboa, Panama. Warkentin, a biology professor at Boston University, conducts her field studies at STRI. That’s where she showed me how she coaxes the eggs to hatch.
The tadpoles leaping from the wet leaf still have a little yolk on their bellies; they probably won’t need to eat for another day and a half. Warkentin keeps rubbing until only a few remain, stubbornly hiding inside their eggs. “Go on,” she tells them. “I don’t want to leave you here all by yourselves.”
The last of the tadpoles land in the water. Predatory bugs known as backswimmers wait at the surface, but Warkentin says she saved the tadpoles from a worse fate. Their mother had missed the mark, laying them on a leaf that didn’t reach over the pond. “If they were hatching on the ground,” she says, “then they would just be ant food.”
***
Warkentin was born in Ontario, and her family moved to Kenya when she was 6. Her father worked with the Canadian International Development Agency to train teachers in the newly independent country. That’s when she got interested in tropical biology, playing with chameleons, and watching giraffes, zebras and gazelles on the drive to school in Nairobi. Her family returned to Canada several years later, but at 20 she went hitchhiking and backpacking across Africa. “That was something that seemed perfectly reasonable in my family,” she says.
Before she started her PhD, she went to Costa Rica to learn more about the tropics and look for a research topic. The red-eyed tree frog’s terrestrial eggs caught her interest. She visited the same pond over and over again, and watched.
“I had the experience—which I’m sure other tropical herpetologists have had before and maybe didn’t think about—if you have a late-stage clutch, if you bump into them, they’ll hatch on you,” Warkentin says. “I bumped into a clutch, and they all were bailing out.”
She had also seen snakes at the pond. “What I thought was, wow, I wonder what would happen if a snake bumped into them,” she says, and laughs. “Like, with its mouth?” Indeed, she found that if a snake appears and starts attacking the clutch, the eggs hatch early. The embryos inside the eggs can even tell the difference between a snake and other vibrations on the leaf. “This is the thing, of going out in the field and watching the animals,” she says. “They’ll tell you things you didn’t expect sometimes.”
Biologists used to think this kind of flexibility got in the way of studying evolution, says Anurag Agrawal, an evolutionary ecologist at Cornell University. No longer. It’s exciting that Warkentin has documented wonderful new things about a charismatic frog, but Agrawal says there’s a great deal more to it. “I think that she gets credit for taking it beyond the ‘gee whiz’ and asking some of the conceptual questions in ecology and evolution.”
What are the advantages of one survival tactic over another? Even a 5-day-old frog has to balance the benefit of avoiding a hungry snake against the cost of hatching early. And, in fact, Warkentin and her colleagues have documented that early-hatching tadpoles were less likely than their late-hatching brethren to survive to adulthood, particularly in the presence of hungry dragonfly nymphs.
Plasticity not only lets frogs cope with challenges in the moment; it might even buy time for evolution to happen. Warkentin has found that tadpoles also hatch early if they’re at risk of drying out. If the rainforest gradually became drier, such early hatching might become standard after countless generations, and the frog might lose its plasticity and evolve into a new, fast-hatching species.
One of the mainstays of evolutionary thinking is that random genetic mutations in an organism’s DNA are the key to adapting to a challenge: By chance, the sequence of a gene changes, a new trait emerges, the organism passes on its altered DNA to the next generation and gives rise eventually to a different species. Accordingly, tens of millions of years ago, some land mammal acquired mutations that let it adapt to life in the ocean—and its descendants are the whales we know and love. But plasticity offers another possibility: The gene itself doesn’t have to mutate in order for a new trait to surface. Instead, something in the environment could nudge the organism to make a change by drawing on the variation that is already in its genes.
To be sure, the theory that plasticity could actually give rise to new traits is controversial. Its main proponent is Mary Jane West-Eberhard, a pioneering theoretical biologist in Costa Rica affiliated with STRI and author of the influential 2003 book Developmental Plasticity and Evolution. “The 20th century has been called the century of the gene,” West-Eberhard says. “The 21st century promises to be the century of the environment.” She says mutation-centric thinking is “an evolutionary theory in denial.” Darwin, who didn’t even know genes existed, had it right, she says: He left open the possibility that new traits could arise because of environmental influence.
West-Eberhard says Warkentin’s group has “demonstrated a surprising ability of tiny embryos to make adaptive decisions based on exquisite sensitivity to their environments.” That kind of variation, West-Eberhard says, “can lead to evolutionary diversification between populations.”
Although not everyone agrees with West-Eberhard’s theory of how plasticity could bring about novelty, many scientists do now think that phenotypic plasticity will emerge when organisms live in environments that vary. Plasticity may give plants and animals time to adjust when they’re dumped in a completely new environment, such as when seeds are blown to an island. A seed that isn’t as picky about its temperature and light requirements might do better in a new place—and might not have to wait for an adaptive mutation to come along.
Also, many scientists think that plasticity may help organisms try out new phenotypes without being entirely committed to them. Early hatching, for example. Different species of frogs vary greatly in how developed they are when they hatch. Some have a stumpy tail and can barely swim; others are fully formed, four-limbed animals. “How do you get that kind of evolved variation?” Warkentin asks. “Does plasticity in hatching time play a part in that? We don’t know, but it’s quite possible.”
***
The town of Gamboa was built between 1934 and 1943 by the Panama Canal Company, a U.S. government corporation that controlled the canal until 1979, when it was handed over to Panama. Gamboa, on the edge of a rainforest, is part ghost town, part bedroom community for Panama City and part scientific summer camp. Quite a few residents are scientists and staff at STRI.
When I visited, Warkentin’s team had up to a dozen people, including several undergraduates she refers to as “the kids.” One morning a posse of vigorous-looking young people in knee-high rubber boots, backpacks and hats departs Warkentin’s lab and strides across the field behind the school, past the tennis courts.
James Vonesh, a professor at Virginia Commonwealth University, who did a postdoctoral fellowship with Warkentin and still collaborates with her, points out his favorite sign in town, a holdover from the Canal Zone era: “No Necking.” It’s painted on the front of the stands at the old swimming pool, now part of the local firefighters’ sports club. Then he explains to one of the kids what “necking” means.
They walk down a road into a nursery for native plants, cross a ditch on a footbridge and arrive at Experimental Pond. It was built of concrete to specifications provided by Warkentin and Stan Rand, a revered frog researcher at STRI, who died in 2005.
On the pond’s far side is the group’s research area, bounded by a ditch on one side and a stream, then rainforest, on the other. There’s a metal-roofed shed with open sides, surrounded by dozens of 100-gallon cattle tanks used in experiments. They look like buckets set out to catch an array of extremely large leaks. Vonesh talks about the plumbing system with more enthusiasm than seems possible. “We can fill a cattle tank in three or four minutes!” he exclaims.
All that fast filling means the researchers can do quick experiments other aquatic ecologists can only dream of. Today they’re dismantling an experiment on predation. Four days ago, 47 tadpoles were put in each of 25 tanks along with one Belostomatid, a kind of water bug that eats tadpoles. Today, they’ll count the tadpoles to find out how many the Belostomatids ate.
A giant blue morpho butterfly flits by, its iridescent wings a shocking splash of electric blue against the lush green forest. “They come by, like, the same place at the same time of day,” Warkentin says.
“I swear I see that one every morning,” Vonesh says.
“It’s the 9:15 morpho,” Warkentin says.
Warkentin explains the experiment they’re finishing today. “We know that predators kill prey, obviously, and they also scare prey,” she says. When new-hatched tadpoles fall into a pond, water bugs are one of the threats they face. The tadpoles’ plasticity might help them avoid being eaten—if they can detect the bugs and somehow respond.
Ecologists have developed mathematical equations describing how much prey a predator should be able to eat, and elegant graphs show how populations rise and fall as one eats the other. But what really happens in nature? Does size matter? How many 1-day-old tadpoles does a fully grown water bug eat? How many older, fatter tadpoles? “Obviously, we think small things are easier to catch and eat and stick in your mouth,” Vonesh says. “But we really haven’t incorporated that into even these sort of basic models.”
To figure out how many tadpoles got eaten, the undergraduates, graduate students, professors and a postdoctoral fellow have to get every last tadpole out of each tank to be counted. Vonesh picks up a clear plastic drink cup from the ground by his feet. Inside is a water bug that was feasting on tadpoles. “He’s a big guy,” he says. He reaches into a tank with the net, pulling out tadpoles one or two at a time and putting them in a shallow plastic tub.
“You ready?” asks Randall Jimenez, a graduate student at National University of Costa Rica.
“I’m ready,” Vonesh says. Vonesh tips the tank as Jimenez holds a net under the gushing water. The guys watch the net for any tadpoles that Vonesh missed. “See anybody?” Vonesh asks. “Nope,” Jimenez says. It takes almost 30 seconds for the water to flow out. Most of the researchers wear tall rubber boots to protect against snakes, but they’re useful as the ground rapidly turns to mud.
A flock of grackles wanders nonchalantly through the grass. “They like to eat tadpoles,” Vonesh says. “They like to hang out and pretend they’re looking for earthworms, but as soon as you turn your back, they’re in your tub.”
Vonesh takes his tub of tadpoles to the shed where Warkentin photographs it. A student will count the tadpoles in each picture. Insects and birds sing from the trees. Something falls—plink—on the metal roof. A freight train whistles from the train tracks that run alongside the canal; a group of howler monkeys barks a raucous response from the trees.
To scientists like Warkentin, Gamboa offers a bit of rainforest about an hour’s drive from an international airport. “Oh, my god. It is so easy,” she says. “There’s a danger of not appreciating how amazing it is. It’s an incredible place to work.”
During the day, the iconic red-eyed frogs aren’t hopping about. If you know what you’re looking for, you can find the occasional adult male clinging to a leaf like a pale green pillbox—legs folded, elbows tucked by his side to minimize water loss. A membrane patterned like a mosque’s carved wooden window screen covers each eye.
The real action is at night, so one evening Warkentin, Vonesh and some guests visit the pond to look for frogs. The birds, insects and monkeys are quiet, but amphibian chirps and creaks fill the air. One frog’s call is a clear, loud “knock-knock!” Another sounds exactly like a ray gun in a video game. The forest feels more wild at night.
Near a shed, a male red-eyed tree frog clings to the stalk of a broad leaf. Tiny orange toes outspread, he shows his white belly and wide red eyes in the light of multiple headlamps. “They have these photogenic postures,” Warkentin says. “And they just sit there and let you take a picture. They don’t run away. Some frogs are, like, so nervous.” Maybe that’s why the red-eyed tree frog has gotten famous, with its picture on so many calendars, I suggest—they’re easier to photograph than other frogs. She corrects me: “They’re cuter.”
Scientists think the ancestors of modern frogs all laid their eggs in water. Maybe the red-eyed tree frog itself could have evolved its leaf-laying habits as a result of phenotypic plasticity. Maybe an ancestor dabbled in laying its eggs out of the water, only on really wet days, to get away from aquatic predators—a plastic way of dealing with a dangerous environment—and that trait got passed on to its descendants, which eventually lost the ability to lay eggs in water at all.
Nobody knows if that’s how it happened. “That was a very long time ago and no longer amenable to those kinds of experiments,” Warkentin says.
But intriguing experiments on another kind of frog—one that might be still navigating the transition between water and land—are underway. Justin Touchon, a former PhD student of Warkentin’s, studies how the hourglass tree frog, Dendropsophus ebraccatus, lays its eggs, which are less packed with jelly and more prone to drying out than red-eyed tree frogs’. A female hourglass tree frog seems to choose where to lay eggs based on dampness. At ponds shaded by trees, Touchon found, they’ll lay eggs on leaves above the water, but at hotter, more exposed ponds, the eggs go in the water.
In a study published last month, he found that eggs were more likely to survive on land if there was a lot of rain, and more likely to survive in water if rainfall was scarce. He also looked at rain records for Gamboa in the past 39 years and found that while overall rainfall hasn’t changed, the pattern has: Storms are larger but more sporadic. That change in the environment could be driving a change in how the hourglass tree frogs reproduce. “It gives a window on what caused the movement to reproducing on land to occur,” Touchon says—a climate that shifted to have lots of steady rain could have made it safer for frogs to lay eggs out of the water.
Warkentin’s group is based on the ground floor of the Gamboa Elementary School, which closed in the 1980s. One morning, Warkentin sits on an ancient swivel chair with dusty arms at a retired office desk, doing what looks like a grade-school craft project.
On the floor at her left sits a white bucket with rows of green rectangles duct-taped to the inside. She reaches down and pulls one out. It’s a piece of leaf, cut with scissors from one of the broad-leafed plants by the experimental pond, and on it is a clutch of gelatinous red-eyed tree frog eggs. She tears off a strip of tape and sticks the piece of leaf onto a blue plastic rectangle, cut from a plastic picnic plate.
“You can do an amazing amount of science with disposable dishware, duct tape and galvanized wire,” she says.
She stands the card in a clear plastic cup with a bit of water in the bottom, where the tadpoles will fall when they hatch, and goes on to the next piece of leaf. The tadpoles will be part of new predation experiments.
There’s great explanatory value in simple models—but she wants to understand how nature actually operates. “We’re trying to grapple with what’s real,” she says. “And reality is more complicated.”