High Hopes for a New Kind of Gene
Scientists believe that microRNA may lead to breakthroughs in diagnosing and treating cancer
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Dr-Carlo-Croce-lab-631.jpg)
I clutch the seat as the Ferrari halts abruptly at an intersection, then purrs impatiently until the light changes. When it takes off, the roar feels oddly extravagant for the quiet streets of suburban Columbus, Ohio.
The driver is Carlo Croce, a 64-year-old Italian scientist with a big voice, disheveled curly hair and expressive dark eyes. He heads the Human Cancer Genetics Program at Ohio State University, and his silver Scaglietti Ferrari is a fitting symbol of his approach to science: grand, high-powered and, these days especially, sizzling hot.
Croce, who grew up in Rome as the only child of a mechanical engineer father and a homemaker mother, went to medical school at the University of Rome and came to the United States in 1970 to study cancer. "I thought it was the place to work in science," he says. Croce was one of the first scientists to establish that cancer—the runaway growth of cells normally held in check—can be caused by genetic changes. He has identified particular gene alterations associated with lung and esophageal cancers as well as with various types of lymphoma and leukemia.
Colleagues say Croce has remarkable scientific instincts. "If you spread out five things in front of him, he can almost unerringly pick the one which is going to work," says Webster Cavenee, director of the Ludwig Institute for Cancer Research in San Diego. "He can smell something interesting, and he's almost never wrong."
It was a few years ago that Croce began to sniff out one of the most surprising and most promising discoveries in cancer research. The discovery placed him and his collaborators at the leading edge of a now-booming field that promises improved techniques for diagnosing disease and, they hope, more effective new treatments. Indeed, Croce's latest work is part of a whole new way of looking at genes and how life regulates itself. Which makes all the more remarkable the fact that his insight came only after he and his co-workers had raced at top speed into a dead end.
One of the glories of 20th-century science was the 1953 discovery of the structure of the genetic material DNA; it is a long ladder-like polymer twisted into a double helix. Each rung is a chain of chemical compounds, called bases, and their exact sequence encodes a gene's instructions, much like the letters in a word. Over the decades, mountains of laboratory evidence led scientists to make two bedrock assumptions about genes.
First, a gene is relatively large, typically consisting of tens of thousands of chemical bases in a row.
Second, the main job of any particular gene is to instruct cells to make its corresponding protein. A protein is a large, complicated molecule that performs a specific function depending on how it's made: it can be part of a muscle fiber or an enzyme that digests food or a hormone that controls physiology, among many other things.
Certainly Croce held these assumptions when, in the early 1990s, he set out to identify a gene involved in chronic lymphocytic leukemia, or CLL. The blood cancer fills the bone marrow and lymph nodes with cancerous cells that crowd out healthy cells of the immune system, leaving the body less able to fight infection. Croce had analyzed cancer cells from people with CLL and found that many were missing the same long segment of DNA. Somewhere on that segment, he reasoned, was a gene crucial to preventing white blood cells from becoming cancerous.
For nearly seven years, Croce and his colleagues kept zeroing in on different bits of that long-suspect strand of DNA, painstakingly determining its genetic sequence, base by base. They also did numerous experiments testing whether the genes could cause CLL.
They struck out. "We characterized every bloody gene present in that DNA and none of it was the gene" associated with CLL, Croce recalls. "I was very frustrated." So were his students and collaborators. "Oh, I burned the lives of a few people," Croce adds. One researcher quit science altogether to get a degree in business administration.
In 2001, Croce hired George Calin, a Romanian gastroenterologist, to take on the project everyone had grown to hate. "He had nothing worse in the lab," Calin jokes.
"Look," Croce told Calin, "the gene has to be there."
Around the same time, a new understanding of genetics was beginning to circulate. Oddly enough, it was facilitated by a mutant worm that was unable to lay eggs. The animal met a ghastly fate: hundreds of eggs hatched inside its body, causing it to burst open. Victor Ambros, a developmental biologist then at Harvard (now at the University of Massachusetts Medical School), was studying the mutation responsible for the worm's genetic defect. The worm, Caenorhabditis elegans, is a microscopic creature that geneticists love to study because it is easy to grow—it eats common bacteria—and is transparent, so all of its 900 or so cells can be observed as they develop. Curiously, as Ambros searched for the mutated gene, the section where it seemingly had to be became too small to contain a normal gene. "It became less and less clear that this piece of DNA could encode a protein," he says. "It was pretty astonishing."
Across the Charles River, at Massachusetts General Hospital, a molecular biologist named Gary Ruvkun was studying a different C. elegans mutant. Ambros and Ruvkun both suspected that the gene Ambros was looking for somehow controlled the gene that had gone awry in Ruvkun's worms. Working on a hunch, they decided to compare the two genes to see if they resembled each other.
"We e-mailed each other our sequences and we agreed to call in later on if we saw anything," Ambros recalls. "One of us called the other one and I said, 'Gary, you see it? And he said, 'Yes, I see it!'" They had found a perfect match—a stretch of DNA from Ambros' short genetic sequence identical to a section of Ruvkun's normal-size gene.
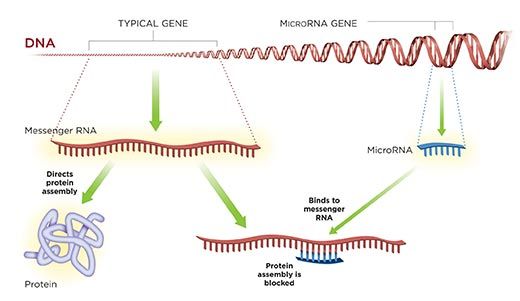
Ambros' gene was truly tiny, only 70 bases long, not 10,000 bases like other genes. Stranger still, the gene didn't make a protein, as other genes do. Instead, it made another kind of genetic material, which is now called microRNA. Traditional genes make RNA also, a molecule that is chemically similar to DNA, but that RNA is short-lived, serving as a mere messenger or intermediary in the construction of proteins. But this microRNA was the gene's end product, and it was no mere messenger.
MicroRNA, Ambros and Ruvkun realized, worked by an intriguing mechanism: it acted like a miniature strip of Velcro. Because the microRNA gene matched part of a traditional gene, the microRNA stuck to RNA produced by the traditional gene. In doing so, it blocked the other gene from producing protein.
It was a fascinating find, but the two scientists thought it was just an oddity until, seven years later in 2000, a researcher in Ruvkun's lab, Brenda Reinhart, found a second microRNA gene in the worm. "That told me that small RNAs were going to be more common than we expected," says developmental biologist Frank Slack, who helped with the discovery in Ruvkun's lab and is now at Yale.
The Ruvkun lab started looking for microRNA genes in other animals. As it happened, it was a great time to search for genetic anomalies. In 2001, scientists completed a draft of the entire sequence of human DNA, known as the human genome, and they were rapidly sequencing other genomes, including those of the mouse, mustard plant, fruit fly and malaria parasite. Some genomes were becoming available on Internet databases, and Ruvkun found the same microRNA gene from the C. elegans worm in fruit flies and human beings. Then he found the gene in mollusks, zebra fish and other species. Meanwhile, Ambros' group and others were finding dozens of additional microRNA genes.
The results were tantalizing—after all, it is not every day that a new class of genes is discovered—but it wasn't clear what role these miniature genes might play in people's lives.
That's when Carlo Croce and George Calin decided to take a fresh look at the mysterious case of the missing leukemia gene. Calin, who is now a molecular biologist at the University of Texas MD Anderson Cancer Center, typed the known microRNA gene sequences into his computer, comparing them with the stretch of DNA that many CLL patients' cancer cells lack. "They were exactly there," he recalls: two microRNA genes sat right where the CLL-suppressing gene was presumed to be.
Calin called Croce into the lab right away: "Dr. Croce, these are the genes!"
Croce looked at Calin and blinked. "S---!," Calin recalls him saying. "These are the genes!'"
Calin and Croce tested blood samples from leukemia patients and found that 68 percent contained little or none of the two microRNAs, while blood cells from people without the cancer had many of the molecules. Calin and Croce were convinced: these two tiny genes made microRNAs that suppressed cancer.
"I was stunned," says Croce. "We had the dogma that all the cancer genes were protein-coding genes," says Croce. MicroRNA "explained a lot that we couldn't explain before. It changed the way we looked at the problem."
Calin and Croce published their finding in 2002—the first time anyone had implicated microRNAs in human disease.
Since then, "every cancer we look at, we find an alteration in microRNA," says Croce. "In probably every human tumor there are alterations in microRNA."
Croce lives in a stately mansion in Columbus' Upper Arlington suburb. Mounds of mail are scattered on the kitchen table when we arrive. Croce has been away from home for weeks, attending conferences and giving talks at the National Institutes of Health in Bethesda, Maryland, the National Academy of Sciences in Washington, D.C., a cancer meeting in San Diego, Johns Hopkins University in Baltimore and three meetings in Italy. The house feels empty and unused.
"Essentially, it's just for sleeping," Croce's son, Roberto, 29, says later about his father's house. "He mostly just parks his possessions there. If he's in town, he's at work, or he hangs out with me." Roberto is working toward a PhD in economics at Ohio State. (Carlo, who has never married, also has a 12-year-old daughter who lives in Buenos Aires.)
Inside the house, art, not science, takes center stage. Croce owns more than 400 paintings by 16th- to 18th-century Italian masters. He built a cavernous 5,000-square-foot wing—21-foot ceilings and all—to display some of the largest paintings.
Croce says he bought his first painting when he was 12 years old, for $100. He likes to buy paintings when he has a suspicion about who the artist is but doesn't know for sure. "I never ask someone," he says. "I just buy it and then I may be wrong or I may be right." He bought one painting for $11,500 from a gallery in Naples. He thought it might be by a Baroque painter named Bartolomeo Schedoni. "I made a picture after it was restored, and sent it to the expert on Schedoni. He said, 'Oh yeah, that is the Schedoni.'" The painting, Croce says, is probably worth 100 times what he paid for it.
"His art collecting has the same experimental bent that his science has," says Peter Vogt, a cancer researcher at the Scripps Research Institute in La Jolla and a friend of Croce's.
Over the years, Croce has patented several discoveries and co-founded three companies. His lab at Ohio State is on the top two floors of a ten-story building. With a staff of about 50 people, the lab has a budget of about $5 million a year, which is on par with a small biotechnology company. His funding comes from federal and private grants.
"There are a lot of people who would say he is entirely successful because he has a huge amount of resources. I actually think it's the other way around; I think he has huge amounts of resources because he is successful," Cavenee says.
As soon as Croce suspected a connection between microRNAs and cancer, he started asking questions: Would cancer cells have different amounts of microRNAs than normal cells have? Would some microRNAs be more common than others in certain types of cancer? "He was really the first person to make that leap," says Slack about Croce's early bet on microRNAs. "It took somebody with Carlos' vision and money to really move the field forward."
In 2003, Croce recruited Chang-Gong Liu, then a microchip developer at Motorola, to design a tool that can test for the presence of microRNAs in a sample of cells or tissues. Using the tool, called a microarray, Croce's laboratory has found microRNAs that seem to be unique to certain types of cancers. For the 3 to 5 percent of patients whose cancer has metastasized, or spread, from an unknown source within the body, the implications of this finding are huge. Because knowing where the cancer began is a key to optimal treatment—tumors arising in different tissues respond to different approaches—microRNAs may be able to help oncologists prescribe the best treatments for such patients.
MicroRNAs may also be able to estimate a cancer's severity. Croce and his collaborators found that the levels of two microRNAs—called Let-7 and mir-155—predicted survival in lung cancer patients. Croce's group has also found microRNAs that predict whether a patient's CLL will become aggressive or stay mild. In the future, a patient's microRNA profile might indicate whether he or she should undergo an aggressive and risky treatment or a milder, safer one.
Today, researchers have identified about 40 microRNA genes associated with cancers, including those of the breast, lung, pancreas and colon. Like conventional genes that produce proteins, microRNA genes can also be cancer promoters, causing the disease if they produce too many microRNAs. Or they can be cancer suppressors; if they are damaged or lost, cancer ensues. Moreover, scientists have begun to understand how microRNAs interact with traditional cancer genes, revealing a complex switchboard of connections that seem to happen inside cells as the disease takes over.
Croce's biggest hope is that microRNAs might one day be used as therapies. "I am convinced, absolutely convinced," he says, "that microRNAs will become drugs." In some recent experiments, he and a colleague have injected microRNAs into mice with leukemia or lung cancer. The injections, he says, stopped the cancer growth.
"The evidence is extremely strong right now" that microRNAs play a fundamental role in cancer," says Slack, "and it's getting stronger and stronger every day."
Cancer is not the only disease in which microRNAs are emerging as important players. Studies now suggest that these miniature genes are involved in immune system function, heart disease, schizophrenia, Alzheimer's disease and Tourette's syndrome. Beyond that, there is a long list of diseases that appear to have a genetic basis, but for which no conventional gene has been identified. Thomas Gingeras, a genome researcher at Cold Spring Harbor Laboratory in New York, believes some of these diseases will ultimately be linked to microRNAs. "I think it's undoubtedly going to be the case," he says.
Perhaps that's because the tiny molecules exert so much influence over the rest of the body. Scientists estimate that humans have around 1,000 microRNA genes, which seem to control the activity of at least a quarter of our 25,000 protein-coding genes. "We are astounded by that number and believe it's a minimum," says Nobel laureate Phillip Sharp of M.I.T., in whose laboratory microRNAs are studied.
No wonder, then, that some scientists express embarrassment and regret that they failed to find microRNA genes sooner—chiefly because they didn't challenge basic assumptions about genes.
"It wasn't a technological issue," says Joshua Mendell, a microRNA researcher at Johns Hopkins. "The technology that's required to study microRNAs is not different from the technology used for the last couple of decades," he says. "It was more of an intellectual barrier."
Even Croce, for all his success, regrets that he didn't recognize microRNAs earlier. In the late 1980s, his team was pursuing a cancer gene in a stretch of DNA that did not code for any proteins. "So we trashed the project," says Croce. Now he knows that the gene was a microRNA. "Bias," he says, "is a bad, bad thing."
Sylvia Pagán Westphal is a writer living in Boston who specializes in covering genetics, biology and medicine.