A Triumph in the War Against Cancer
Oncologist Brian Druker developed a new treatment for a deadly cancer, leading to a breakthrough that has transformed medicine
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Druker-cancer-with-patient-631.jpg)
There’s a photograph of LaDonna Lopossa that helps tell the story. She’s all smiles, lying on the grass in a vaguely Betty Grable manner atop her own cemetery plot. The portrait was her husband’s idea—in their decades together it seems George, a.k.a. Mr. No Serious, never saw a gag he didn’t like—but it was LaDonna who came up with the cheesecake pose.
“OK,” George had said, “now take off your shirt.”
“George!”
Click.
On the one hand it’s a silly snapshot of a 60-year-old woman in a cardigan and sensible sandals in Winlock, Washington, one sunny day in May 2000. On the other hand it’s a glimpse of a possible future in which science has solved a fearsome problem. For this is how LaDonna and George faced her lethal cancer, not just whistling past the graveyard but clowning around in the middle of it.
Three months before, LaDonna was lying in a hospital bed in Olympia about to draw the curtain. There was a lot to let go of: four grown children, several grandkids, friends at church, a good marriage. (Never mind that as she lay there George was loudly telling the nurses he was going to hit the bars to find another wife, which she understood as his oddball effort to ease her mind.) She was ready to leave everyone and all those things and more because of the pain.
Her spleen, normally tucked beneath the lowest left rib and no bigger than a peach, was so engorged with white blood cells it was the size of a cantaloupe. She could hardly walk. Her skin was ghostly, her blood dangerously short of red cells. To breathe was a chore. Regular vomiting. Stabbing aches deep in her bones, where the marrow was frantically cranking out white cells, or leukocytes. Recurring fevers. And cold, strangely, unnervingly cold: she was freezing under the hospital blankets.
She was too old and too sick to undergo a bone marrow transplant, a grueling, highly risky treatment for her blood cancer, chronic myeloid leukemia (CML). She had already tried the other standard CML treatment, regular doses of the powerful compound interferon. But it so intensified her nausea, fevers and bone pain she abandoned the medication, come what may. With nothing left in their leukemia-fighting arsenal, the doctors were down to Dilaudid, a derivative of morphine, the narcotic painkiller. It was calming, it was comforting and for a patient in her condition it was, of course, the end.
George had given away most of her belongings and had reserved a U-Haul truck to cart his stuff to Southern California, where he would move in with one of their sons. The music for her funeral was chosen, including “Because I Have Been Given Much,” to be sung by the grandkids. When the hospital recommended moving LaDonna to a hospice, George took her home instead and followed her doctor’s advice to summon the children; Terry, Darren and Stephen flew up from the Los Angeles area, and Kelly drove over from her place in Winlock. One by one they went into the bedroom, sat at LaDonna’s bedside and said goodbye.
CML is one of the four main types of adult leukemia, but it is not common, striking 5,000 people in the United States each year. As a rule, it is fatal, with most patients dying within five years of being diagnosed. The first phase, a stealthy explosion of otherwise normal white blood cells, can last months or years; patients are often alerted to the condition by a routine blood test. If the disease goes unchecked, the white cells become increasingly abnormal, issuing helter-skelter from particular stem cells in bone marrow called myeloid cells; such leukocytes burst capillaries, overwhelm organs and suffocate tissues by crowding out oxygen-carrying red blood cells. The disease’s course is exceptionally predictable, physicians say, but its clockwork nature has also provided scientists with an opportunity: prying into the molecular gears and springs that propel CML, they understand it better than any other cancer.
Once, in early December 1999, George was driving to see LaDonna at the hospital in Olympia and stopped at a Safeway to buy a newspaper. Mr. No Serious is an avid reader, had even briefly run a bookstore with LaDonna, and he devoured the paper in her hospital room. As it happened, an experimental leukemia treatment was then making headlines. “Leukemia Pill Holds Promise,” the Associated Press reported, saying CML patients “had normal blood counts within a month of beginning treatment.” The study was then underway at the Oregon Health & Science University (OHSU) in Portland.
George hurried out of the hospital room to find LaDonna’s oncologist.
Target for Intervention
A steep, winding, tree-lined road leads to the main campus, which is perched near the summit of 574-foot-high Marquam Hill and on foggy days appears to float above the city like a castle in a fairy tale. Another route up to OHSU is the Portland aerial tram: two Swiss-made gondola cars of gleaming steel soar on cables high over Interstate 5, whizzing people back and forth between the west bank of the Willamette River and a hospital platform perched closer to the edge of a cliff than disembarking heart patients might wish it to be.
Brian Druker arrived at OHSU in 1993, years before the tram would be built and the hall-of-fame mural in the adjacent passageway would include a picture of him. Tall, as lanky and lightfooted as a greyhound, soft-spoken, Druker was 38 and had just spent nine years at the Dana-Farber Cancer Institute, part of Harvard Medical School, in Boston. “I saw cancer as being a tractable problem,” he recalled of the research path he chose after finishing medical school at the University of California, San Diego. “People were beginning to get some hints and some clues and it just seemed to me that in my lifetime it was likely to yield to science and discovery.”
At Dana-Farber, Druker landed in a laboratory studying how a normal human cell gives rise to runaway growth—malignancy. Among other things, the lab focused on enzymes, proteins that change other molecules by breaking them down (gut enzymes, for example, help digest food) or linking them up (hair follicle enzymes construct silky keratin fibers). Enzymes also figure in chain reactions, with one enzyme activating another and so on, until some complex cellular feat is accomplished; thus a cell can control a process such as growth or division by initiating a single reaction, like tipping the first domino. Under the lab’s chief, Thomas Roberts, Druker mastered numerous techniques for tracking and measuring enzymes in tissue samples, eventually turning to one implicated in CML.
Working out the details of why this particular enzyme is the key to CML had involved hundreds of scientists around the world—research that would lead to several Nobel Prizes—but here’s basically where Druker started:
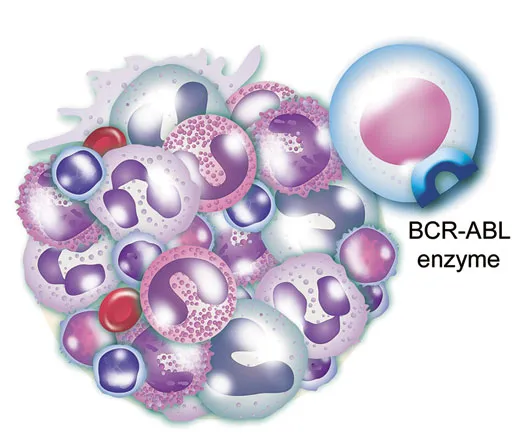
First, all CML patients have the renegade enzyme in their white blood cells.
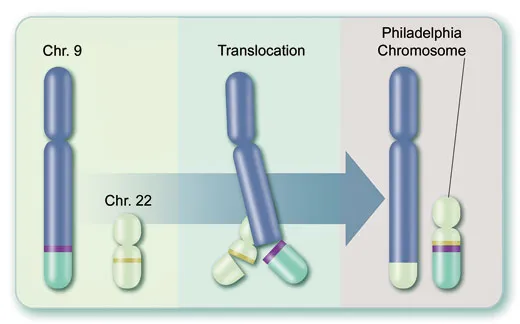
Second, the enzyme itself is the product of a freakish gene, called BCR-ABL, formed during a single myeloid stem cell’s division and thereafter transmitted to billions of descendants: the tips of two chromosomes, those spindly structures that store DNA, actually swap places, causing separated genes called BCR and ABL to fuse (see illustration). The new mutant BCR-ABL gene sits on a peculiar chromosome discovered in 1960 by scientists at the University of Pennsylvania. This “Philadelphia chromosome,” visible through a microscope, is CML’s hallmark.
Third, the BCR-ABL enzyme is the evil twin of a normal enzyme that helps control the production of white blood cells. But like a switch stuck in the “on” position, the mutant spurs the wild proliferation that is leukemia.
You didn’t have to be a Harvard doctor to see that a single enzyme that causes a fatal leukemia was, as researchers say, an attractive target for intervention. And, indeed, scientists were then setting out to find or invent compounds that could block the BCR-ABL enzyme.
Druker and his Boston co-workers, using specially designed antibodies, developed a new way to measure the enzyme’s activity—a tool that would prove invaluable to evaluating potential CML treatments. A necktie-wearing physician among jean-clad PhDs, Druker was racing competitors at other research centers to find a drug that suppresses cancer by disabling a critical enzyme and spares healthy tissues in the bargain. By tradition, cancer treatments carpet-bombed the body with powerful drugs, killing healthy and cancer cells alike—“cytotoxic chemotherapy,” doctors call it. The alternative, targeted therapy, would fight cancer better with less collateral damage, or at least that was the notion that often kept Druker in the lab until 11 p.m.
Then things began to fall apart. “My marriage had broken down. I wasn’t what you would call a devoted husband. I was a devoted researcher and scientist and physician. And that took a toll.” (Druker and his wife split after two years of marriage and were later divorced.)
Still, with a score of published studies and a nifty enzyme-measuring technique to show for his efforts, Druker thought he was ready to move up the Harvard ladder from instructor to assistant professor. “I sat down with the head of medical oncology at Dana-Farber,” Druker recalled. “He looked over my résumé and said, ‘I just don’t think this work is going to go anywhere here.’” Translation: “I was told I had no future at Dana-Farber.”
“It was awful,” he recalled. “I was depressed. But it forced me to really say, Do I believe in myself? Am I going to make it, make a difference?”
Growing Concern
Asked to describe Druker’s approach, one scientist said it boiled down to “perseverance and stubbornness in not letting go of an idea.”
“I think intrinsically he’s a shy person,” said another. “But on this”—cancer therapy—“he’s like a crusader.”
“He takes everything that is complicated, shoves it in his mind and outputs the simplest possible interpretation and intervention.”
“When you ask a question, there’s silence in the room, almost uncomfortable silence, and you’re, like, did he even hear me? He thinks things through before giving an answer.”
“He lets the science do the talking.”
Druker grew up the youngest of four children in St. Paul, Minnesota, and attended public schools, excelling at math and science. His father was a chemist at 3M whose work on printing processes was patented. His mother was a homemaker who got involved in school-board politics and ran unsuccessfully for the state legislature. After graduating with a chemistry degree from UC San Diego, he stayed on, and in 1978, his first year in medical school, he wrote a 16-page paper hinting at a future he would help create. Written in longhand with blue ink on lined notebook paper and titled “Cancer Chemotherapy,” it concluded that, someday, when the action of cancer drugs is “understood in biochemical terms the field of cancer chemotherapy should make advancements far beyond the progress already made.”
After the Dana-Farber Cancer Institute gave him the bum’s rush, Druker marshaled new resolve. “When I moved here to Oregon, my goal was to identify a drug company that had a drug for CML and get that into the clinic,” he said.
He’d previously met Nick Lydon, a biochemist at the Swiss pharmaceutical firm Ciba-Geigy (which would merge with Sandoz in 1996 to form Novartis). Lydon had collaborated with Roberts, Druker’s former lab chief. “I called my friend Nick at Ciba-Geigy and he said, ‘We have what you’re looking for.’” It was called STI571. Company chemists had synthesized it and other compounds while searching for a new anti-inflammatory drug, but they had learned it could also block the activity of enzymes in a test tube. Still, they hadn’t quite decided what to do with the compound.
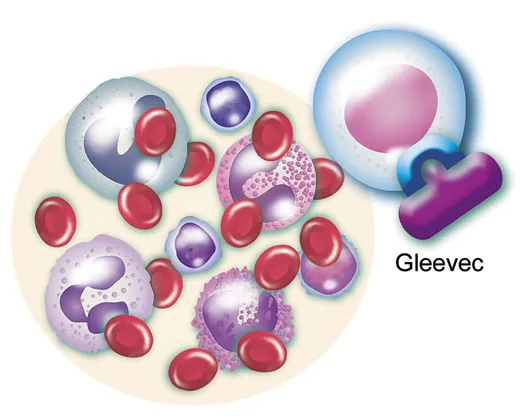
In August 1993, Druker received his first batch of liquid STI571 and another candidate compound from Switzerland. Using the enzyme-measuring tool he’d helped develop, he confirmed that STI571 strongly inhibited the BCR-ABL enzyme, which belongs to a class of enzymes known as tyrosine kinases; the other compound did so only weakly. He also poured minute amounts of STI571 into a tray of thimble-size containers that held fluid and live white blood cells derived from a CML patient. Druker had hoped the cells’ growth would slow or stop. Even better, the cells died. Moreover, a large amount of STI571 given to healthy cells in a dish did no harm. “Brian’s contribution was critical,” Lydon recalled, in convincing the company to “move in that direction.”
But, of course, the road to dashed hopes is paved with experimental drugs that looked terrific in a test tube but failed in human beings. Skeptics pointed out that hundreds of different types of tyrosine kinase enzymes are at work in the body, and, they added, wouldn’t a drug that blocked one also block many others and wreak physiological havoc? “There were many naysayers who argued that it would be impossible to develop specific protein kinase inhibitors” for treating cancer, Tony Hunter, a biochemist at the Salk Institute in La Jolla, California, wrote in the Journal of Clinical Investigation.
Scientific ideas don’t take root like dandelion seeds wafted onto fertile ground. They need advocates, people who want to win. Druker plugged away, doing more experiments, such as inducing a form of CML in laboratory mice and subjecting them to STI571. It all but eliminated the animals’ disease. “I was putting in probably 60 to 80 hours a week,” recalled Druker, who in his scant free time competed in bicycle races, a sport that demands a high tolerance for pain and a sense of when to break out of the pack. “My life in those days was I’d work [in the lab], work out, eat and sleep.” What was driving him, he said, were CML patients who were dying.
By 1997, having published numerous studies with co-workers in Portland and Switzerland, Druker believed the compound was ready to be tried in human beings. Novartis disagreed. For one thing, when dogs had been given the drug in intravenous form, it tended to cause blood clots at the end of the catheter. Novartis chemists spent months reformulating the liquid drug as a pill. But when the researchers gave large doses to dogs, the animals showed signs of liver damage. Some company officials, Druker recalled, advised dropping the project altogether.
But the canine liver damage didn’t faze him; chemotherapy, after all, is destructive. “We knew how to give people toxic cancer drugs,” he said.
The next thing Druker did may not have been illegal, but it certainly wasn’t kosher. He bypassed Novartis and went straight to the Food and Drug Administration to see if he’d accumulated enough data to start a human trial. “I called up the toxicologist at the FDA and said, ‘Here’s the problem.’ And he said, ‘My goodness, you have a ton of data, we would probably accept this application.’” Druker then told Novartis what he’d done. “I got myself in some hot water because I’d gone behind their back.”
Finally, in June 1998, with FDA permission to proceed, Druker administered STI571 to a human being, a 68-year-old Oregon man with CML. “It was almost anticlimactic,” Druker recalled, “in that we’d been ready in November 1996 and here it was over a year and a half later.”
He had recruited two eminent oncologists to help run the clinical trial, Moshe Talpaz at the M.D. Anderson Cancer Center in Houston and Charles Sawyers at UCLA. All the CML patients enrolled in the three cities had undergone interferon therapy and either had failed to improve or had relapsed. None was eligible for a bone marrow transplant.
Gradually increasing the STI571 dosage, the physicians observed by around six months that astronomical white blood counts of nearly 100,000 cells per cubic millimeter were falling to less than 10,000, well within normal. Analysis of one of the first patients’ white blood cells found no signs of the Philadelphia chromosome, suggesting the leukemia had been stopped at the source. More impressive, whatever trace of the BCR-ABL gene remained had ceased copying itself. “That’s when we knew we had something the likes of which had never been seen before in cancer therapy,” Druker said.
As word spread on the Internet, other CML patients wanted in. Druker pressed Novartis to produce more of the drug. But Novartis wasn’t ready. The drug was difficult to make, Daniel Vasella, then the Novartis chief executive officer and now chairman of the board, would recall in his book about the drug, Magic Cancer Bullet. “Nor was [the drug] a high priority, given the small number of CML patients,” he added. Plus, proving that it was both safe and effective would require a substantial investment. “A severe side effect could develop in one out of 1,000 patients and that would be the end of the trial,” he wrote.
In September 1999, Druker got an e-mail from a 33-year-old CML patient in Montreal, Suzan McNamara. She’d been on interferon, which had suppressed her disease for nearly a year, but now it was roaring back, and she wanted to join an STI571 trial. “I was sick to the point where I could barely leave my house,” she recalled to me.
Druker phoned her the next day and said it would be months before she could enroll in a study—Novartis had not committed to producing more STI571. But, he added, the company might move more quickly if it heard directly from patients.
McNamara and a friend used an Internet site to create a petition requesting that the drug be made more widely available; thousands of CML patients endorsed it. She sent it to Vasella with a letter saying, “We have viewed with growing concern our belief...that the supply of the drug has not been sufficient to expand the trials as fast as the evidence to date would warrant.”
“The letter could not be ignored,” Vasella has said. The company increased STI571 production.
The honor of announcing the early clinical results fell to Druker. In New Orleans on December 3, 1999, he told an auditorium full of hematologists that all 31 patients in the study responded favorably to STI571, with the white blood cell counts of 30 falling to normal within a month. The pill’s side effects—upset stomachs, muscle cramps—were what oncologists term “mild to moderate.” Druker says he doesn’t remember the standing ovation.
The findings were “a molecular oncologist’s dream come true,” wrote Harold Varmus, who now heads the National Cancer Institute and was awarded a Nobel Prize for research that laid some of the groundwork for STI571’s success. The drug, he recalls in his 2009 book, The Art and Politics of Science, was “the best evidence to date that the most fundamental aspects of cancer research had dramatic benefits for patients with cancer.”
CNN, the New York Times, “Good Morning America” and the Associated Press covered the breakthrough cancer pill.
Wave of the Future
After LaDonna Lopossa and her children said their goodbyes in February 2000, she eked out a few more days and made it to an appointment at OHSU. LaDonna’s oncologist and George had managed to get her into the second phase of the STI571 trial, which would enroll some 500 new patients at a dozen medical centers worldwide. She shuffled into the clinic on George’s arm. “What have we gotten ourselves into?” one of the nurses said, meaning LaDonna’s death, which appeared imminent, would count as a black mark against the drug. Her white blood count exceeded 200,000, more than 20 times normal. “There were no two ways about it,” Druker said. “You looked at her and she was in trouble.”
They examined her and gave her an STI571 pill. She threw it up.
The next morning, George and LaDonna awoke in her sister’s apartment in Portland and George made LaDonna a banana milkshake. Later that day, the STI571 pill stayed down. And the next, and so on.
“Within three weeks her spleen was back to practically normal,” Druker said. “She was feeling great. White count had come down. A Lazarus-like effect. It was truly miraculous.”
It was in May of that same year that LaDonna and George visited the cemetery in Winlock to place flowers on her mother’s gravesite, which is next to the plot LaDonna had bought for herself. “I’m supposed to be in that grave,” she said to George.
“Well,” he said, “since you’re not, why don’t we take a picture?”
By the late winter of 2001, Druker and his collaborators had pooled much of their STI571 data: in roughly 95 percent of patients, white blood cell levels had returned to normal, and in 60 percent the Philadelphia chromosome was not detected. The company submitted the results with its new-drug application to the FDA, which it approved in two and a half months—to this day the fastest drug review in the agency’s history.
Ten years ago this month, the U.S. government announced that the drug, which Novartis named Gleevec in the North American market (Glivec in Europe), would be available to CML patients. It was a defining moment. The previous century of cancer treatments—intermittently successful, based on trial-and-error testing, almost always agonizing—would be known to experts as “before Gleevec.” From then on was “after Gleevec,” the era of targeted therapy. At a Washington, D.C. press conference on May 10, the Secretary of Health and Human Services, Tommy Thompson, called the drug a “breakthrough” and “the wave of the future.” The then director of the National Cancer Institute, Richard Klausner, described it as “a picture of the future of cancer treatment.”
Today, Suzan McNamara would agree that future is good. When she first traveled to Portland in 2000 to take part in the Gleevec study, she recalled, “I went there with half my hair, and anorexic, and couldn’t even walk up a flight of stairs. And I came back in one and a half months 20 pounds heavier and full of life.” Her next steps were to attend McGill University, study leukemia therapies and earn a PhD in experimental medicine. Now 44, she lives in Montreal and works in Ottawa for Health Canada, a federal agency. Still on Gleevec, she runs several miles a few times a week. “I’d go more if I wasn’t so lazy,” she said. In January 2010 she wed her longtime boyfriend, Derek Tahamont, in Hawaii. “He stood by me through the whole illness and everything,” she said. “We decided to hop on a plane and get married on a beach, just the two of us. It was perfect.”
Gleevec has encouraged people to think cancer is not always a deadly invader that must be annihilated but a chronic ailment that can be managed, like diabetes. In follow-up studies led by Druker, some 90 percent of newly diagnosed CML patients who began taking Gleevec had survived five years. “I tell patients how optimistic I am about their future,” Druker said. “We’re projecting for Gleevec that average survival will be 30 years. Someone who’s diagnosed at 60 can live to 90, and die of something else.”
Back when LaDonna Lopossa was 60, she recalled, Druker said he would keep her alive until she was 70. Then she reached that milestone. “I meant when I turned 70,” he joked to her then.
LaDonna, now 71, and George, 68, live in Battle Ground, Washington, a rural town 24 miles north of OHSU, where LaDonna remains under Druker’s care. The Lopossas live in a bungalow in a state-subsidized senior-citizen housing complex across the street from a family that keeps hens in the yard and lets George grow herbs. A framed magazine ad for Gleevec featuring LaDonna hangs on a living room wall. Two portraits of Christ grace a dining room wall. George, who is quick to say he’s not religious—“nobody knows what Jesus looked like,” he quipped of LaDonna’s iconography—has his own den, where he watches “Family Guy.”
LaDonna volunteers at the North County Community Food Bank down the street, at the Mormon church she belongs to and, by telephone, she counsels people newly diagnosed with CML for the Leukemia and Lymphoma Society. One of her biggest challenges these days, she said, is convincing patients to keep taking Gleevec; they haven’t endured the symptoms of fulminating CML and some find the drug’s side effects annoying.
Gleevec held LaDonna’s CML at bay for seven years, at which time her disease became resistant to the drug. Fortunately, medical scientists and drug companies had developed two new CML drugs, each disabling the BCR-ABL enzyme in a different fashion and compensating for a type of Gleevec resistance. Sprycel didn’t help LaDonna, but Tasigna did—for about two years. Now she’s on her fourth targeted CML drug, bosutinib, which is still experimental. “Her leukemia is the best controlled it’s ever been since I have taken care of her in the past 11 years,” Druker said.
Personalized Oncology
Seated at the small round conference table in his small corner office high on Marquam Hill, Druker said he was still studying CML, hoping to understand how to eliminate every last mutant stem cell, and he was also trying to apply “the Gleevec paradigm” to other leukemias. A bright yellow bicycle-racing jersey worn and autographed by the Tour de France champ and cancer survivor Lance Armstrong hung framed on the wall. It was a clear day and the great vanilla ice-cream scoop of Mount St. Helens was visible out the window facing north and the storybook white triangle of Mount Hood could be seen through the window facing east. The guy who didn’t have the right stuff to be a Harvard assistant professor is today the director of OHSU’s Knight Cancer Institute, named after Phil Knight, the founder of Nike and a Portland native, and his wife, Penny, who in 2008 pledged $100 million to the facility. “Brian Druker is nothing short of a genius and a visionary,” Phil Knight said at the time.
The honors have poured in, including the field’s top U.S. prize, the Lasker-DeBakey Clinical Medical Research Award, which Druker shared in 2009 with Lydon and Sawyers. Of his many appearances in the news media none would change his life more than a story about him in People, “The Miracle Worker,” published in February 2001. The magazine had sent a reporter named Alexandra Hardy to interview the dragon-slaying physician at the hospital in the clouds. The two were married in 2002 and are parents to Holden, Julia and Claire. Said Druker: “I have the ability now to focus on family as a priority. I couldn’t have done that 10 or 15 years ago.”
To some observers, the Gleevec fable soon lost its luster. “‘Wonder Drug’ for Leukemia Suffers Setback,” the Wall Street Journal reported in 2002 once some patients became resistant to the drug or could not tolerate it. Also, it seemed researchers were slow to produce other drugs targeted to tame other cancers, calling the strategy’s promise into question. A Time reporter blogged in 2006 that Gleevec was a “Cinderella drug”—a glass slipper that fit a singular candidate. Sawyers said he got tired of researchers saying Gleevec was a one-off, a lucky shot.
The drug’s cost has been controversial since Day 1. A year’s supply in the United States now runs about $50,000, or around $140 per daily pill. That is twice the original cost, which Vasella had defended as “high” but also “fair,” because the drug gives patients a good quality of life and the company’s revenue underwrites research on other drugs. (Asked about the reasons for the price increase, a Novartis spokeswoman declined to comment.) In any event, a drug that Novartis balked at developing because the market was too small is now a blockbuster. In 2010, Gleevec generated $4.3 billion in worldwide sales—the company’s second-highest-grossing drug. To be sure, Novartis has provided free or discounted medication to low-income patients. In 2010, the company assisted some 5,000 U.S. patients by donating to them $130 million worth of Gleevec and Tasigna, also a Novartis drug.
But patients, doctors and others have long complained about Gleevec’s price. In her 2004 book, The Truth About the Drug Companies, Marcia Angell, former editor of the New England Journal of Medicine, suggested Novartis was “gouging” patients on Gleevec. Recently, physicians have reported that patients stopped taking Gleevec because they could not afford it, despite the company’s assistance program.
Druker, who said his lab has received Novartis research funding but neither he nor OHSU has ever earned Gleevec royalties, deplores the cost. “It should be an affordable price, which would be in the $6,000 to $8,000 a year range,” he told me. “The company would still have plenty of profits.” He went on, “Many cancer drugs are now priced well out of the realm of affordability. As a health care industry, we’re going to have to tackle and deal with that.”
There will be plenty to deal with: it appears Gleevec was not merely a lucky shot. Just the fact that scientists quickly designed new drugs to cope with Gleevec resistance shows they increasingly know what they’re doing, said Sawyers, now at Memorial Sloan-Kettering Cancer Center. He led a group that was the first to explain resistance and was involved in Sprycel’s development. “Why am I so optimistic?” he said. “We know the enemy and we know how to vanquish it.”
Indeed, several enzyme-targeted cancer therapies won FDA approval in Gleevec’s wake, including drugs against particular forms of lung cancer and pancreatic cancer. And researchers say they’re heartened by treatments well along in clinical trials. Some melanoma patients whose disease is caused by a known genetic mutation appear to benefit greatly from an experimental drug called PLX4032. Sawyers is studying a form of prostate cancer spurred by a mutant hormone receptor, and he said clinical tests of a drug (called MDV3100) targeted against it are “exciting.” One pharmaceutical-industry analysis estimates that drug companies are currently developing and testing nearly 300 targeted molecular cancer therapies à la Gleevec.
Arul Chinnaiyan, a research pathologist specializing in cancer at the University of Michigan Medical School, in Ann Arbor, is frank about Gleevec’s influence. “We’re trying to franchise its success,” he said of his attempts to apply the targeted-therapy approach to solid tumors, which are more complex than CML. Each type of solid tumor may be driven by multiple errant enzymes and receptors—protein structures that transmit chemical messages—and the variety of mutations might vary person to person. Chinnaiyan himself has discovered two different mutant gene fusions analogous to BCR-ABL that appear to drive many prostate cancers. “The thought is if we know these are the molecular lesions, we’ll be able to match the drug or combination of drugs appropriately,” Chinnaiyan said.
I got a sense of what he calls “personalized oncology” one day in a brew pub in Ann Arbor. Across the scarred wooden table eating a bacon cheeseburger and sipping ale was Jerry Mayfield, 62, a former Louisiana state trooper. Diagnosed with CML in 1999, Mayfield was told at the time by his hematologist that he had two to three years to live. Mayfield asked if there were experimental drugs to consider. The doctor said no. Mayfield checked the Internet, learned about STI571 and, having taught himself computer programming while manning the night desk at police headquarters in Monroe, created a Web site, newcmldrug.com, to inform other patients. If he’d listened to his hometown doctor, Mayfield said, “without question I would not be here today.”
He still runs his Web site, and these days lives in Bloomington, Illinois. He was in Ann Arbor to see Talpaz, who had collaborated on the initial Gleevec clinical trials in Houston but had moved to the University of Michigan. He has taken care of Mayfield for more than a decade, administering targeted therapies in succession as Mayfield became resistant or could no longer tolerate them: Gleevec, Sprycel, Tasigna, bosutinib and now ponatinib, yet another experimental kinase-blocking CML drug racing through clinical trials.
Mayfield is “a poster boy for CML therapy,” Talpaz told me. “He’s doing extremely well.”
Over the pub’s blaring music Mayfield said of his BCR-ABL gene, “I had the G250E mutation—have the G250E mutation—which is why I became resistant to Gleevec.”
His remark sounded like something out of a time machine programmed to years or decades from now, when people will nonchalantly talk about their deadly genetic mutations and the drugs that stymie them. It’s an image Druker often conjures. “In the not-too-distant future,” he wrote when accepting the Lasker-DeBakey Award, “clinicians will be able to thoroughly analyze individuals’ tumors for molecular defects and match each person with specific, effective therapies that will yield a durable response with minimal toxicity.”
Mayfield has never been treated by Druker but has consulted him. “I was sitting in my local oncologist’s office one day ten years ago, and my cellphone rang,” Mayfield said. “It was Dr. Druker. I’d sent him an e-mail. I was stunned. I told my oncologist, ‘It’s rude to answer this call but this is my hero.’ He’s such a kind and gentle and dedicated man, not the least bit arrogant. He has saved so many lives. Everybody in the country should know his name. He’s the kind of idol we should have, instead of sports stars.”
Mayfield’s Web site has an “appreciation album” dedicated to Druker, filled with tributes from CML patients. Snapshot after snapshot shows people smiling in bright sunlight—hiking, planting trees, drinking champagne—people who felt moved to say they owed him, well, everything. They submitted dozens of poems and limericks, such as this one by a patient named Jane Graham:
There once was a doctor named Brian
On whose research we all were relyin’
He knew we were ill,
So he made us a pill,
And now we’re not plannin’ on dyin.’
Contrary to Expectations
Druker met with LaDonna Lopossa in the examining room where he sees study patients every Thursday. George, who says LaDonna has an “unsinkable-Molly Brown quality,” had driven her down from Battle Ground for her checkup. She sat in a chair while Druker, wearing a loose-fitting dark blue suit, leaned against the edge of an examining table. “I wouldn’t be here without you,” LaDonna said (possibly for my benefit).
“Well, you’re here,” Druker said. “You’re doing well.”
“I’m, like, dancing-in-the-streets well.”
“Great. Any problems?”
“No. I just have a rash.”
“When did that start?”
“About ten weeks ago.”
He asked about the rash, and later I would leave the room so he could examine her.
“You still working at the food bank?” he asked.
“I’m doing one day a week.”
“How’s that going?”
“Terrific.”
“How’s your energy?”
“My energy is low. But my brain is active.”
“You’re just doing spectacularly, leukemia-wise.”
“I know it. I can feel it.”
“What else? Questions for me?”
“I’m going on a trip tomorrow.”
“To?”
“San Diego and Knott’s Berry Farm with all my grandkids.” She updated their progress, and Druker recited their ages, as if to check that he had the facts right. When he addresses scientists at professional conferences, he often shows photographs of LaDonna and her grandchildren. Contrary to all expectations, he says, she is getting to watch her great-grandchildren grow up.
“I have such a wonderful life,” LaDonna said, tearing up. “And I didn’t want it. I told my doctors, ‘Don’t do any more to me.’ ”
Dabbing her eyes with a tissue, she mentioned her first visit to the clinic, in 2000, when she’d barely made it through the door. “That was a long time ago,” she said to no one in particular.
Then, to Druker, she said, “But it’s gone fast, hasn’t it?”
“Hasn’t it?” he said.
Terence Monmaney first wrote for Smithsonian in 1985. He is the executive editor. Portland-based Robbie McClaran photographed his adopted hometown for the November 2010 issue.