Is This New Material a Game Changer for Thermoelectricity?
Researchers at the University of Utah have developed an inexpensive, non-toxic material that converts heat to electricity
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/38/08/38082214-6741-4f97-9956-e11658024436/u-of-utah-thermoelectricity.jpg)
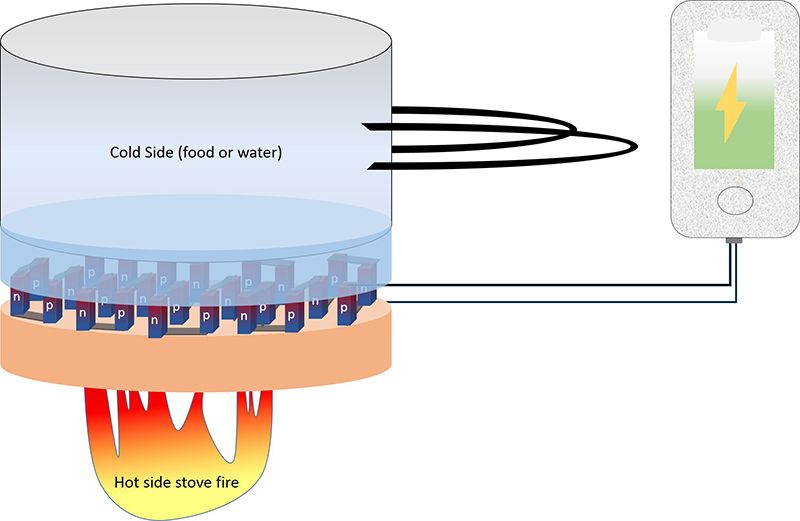
You hike to an elusive camping spot, pack filled with enough gear to keep you content for a three-day retreat away from chaotic city living. But when you’re ready to leave, you realize not only has your cell phone died, its battery spent after searching for a signal the entire time you’ve been roughing it, but you can’t quite remember where you hiked in, which means that the GPS on your phone is your lifeline back to reality. Fortunately, because of a new material built into your cooking pot, all you need to do is turn the pot on, heat up the water inside and plug your phone into the port connected to it. In only a few hours, your phone will be charged and you can make it safely back to your truck parked at the trailhead.
Researchers at the University of Utah recently discovered that the non-toxic material composed of three chemical elements—calcium, cobalt and terbium—generates thermoelectric energy from waste heat. By sandwiching the Ca3Co4Og between a layer that is hot, such as a cooking pot, and a layer that is cold, like the food or water within the pot, the charge from the hot end moves through the cold end, producing an electrical voltage.
The energy is generated through a thermoelectric process using temperature differences. In this case, materials science and engineering post-doc researcher Shrikant Saini says, even one degree of temperature difference produces a detectable voltage.
“In thermoelectric materials, when one end of the material is hot and the other end is cold, charge carriers from the hot end move through the material to the cold end, generating an electrical voltage,” says Saini, lead author on the paper recently published in Scientific Reports. “A few milligrams of this material will provide roughly a microwatt of electricity.”
Because the material is such a new discovery, Saini says that they are in the middle of analyzing the exact grams to watts measurement; however, their rough estimate shows that for one watt of power to be generated, they need about five grams of the material.
An old proverb cautions us to “waste not, want not.” But waste—energy waste—is tricky to capture. In the U.S., nearly half of our energy is lost due to inefficiency, and the majority of our energy is still generated from non-renewable petroleum, natural gas and coal. According to a U.S. energy chart assembled by the Lawrence Livermore National Laboratory, of the 97.4 quadrillion British thermal units (or quads) of raw energy generated in 2013 from solar, nuclear, hydro, wind, geothermal, natural gas, coal, biomass and petroleum, only 38.4 quads were actually used. That means 59 quads were wasted. Finding a way to collect and use this wasted energy could provide a sustainable resource for the future.
“Waste heat is indeed a largely overlooked, yet vast reservoir of possible energy,” says Jeffrey Urban, inorganic facility director at the Molecular Foundry at Berkeley Labs. “Thermoelectrics are a promising route to harness and take advantage of this resource—they directly convert heat to electricity with no moving parts, working fluids or other mechanical complexity.”
Urban notes that efficiency, costs of materials and ease of implementation are all important engineering considerations, adding, “Due to the complex transport physics, thermoelectrics tend to operate optimally at only one particular temperature.”
Previous thermoelectric material compositions were made up of cadmium, telluride or mercury—elements that were all toxic to humans and, according to Saini’s research, not as stable as the Ca3Co4Og combination. Also, prior thermoelectric materials were not scalable because they were derived from manufacturing or fabricating single crystals, which is both expensive and challenging. Saini’s chemical combination may allow for large-scale application of this thermoelectric technology because the chemicals are readily available to mix up and cook to derive the non-toxic material, making it easier to manufacture in larger batches. This makes the discovery a possible game changer.
“We anticipate many applications of this material,” says Saini. The University of Utah has applied for a patent. Saini is unable to reveal some specific details, but adds that the newfound material could be used in jewelry, cooking pots and automobiles—or even have future medical applications.
Thermoelectricity—or electricity produced through temperature differences—originated in 1821 when Thomas Seebeck and Jean Peltier discovered the conversion of heat to electricity. Three decades later in 1851, William Thomson (also known as Lord Kelvin) discovered that running an electric current through a material can heat or cool it, depending on how the electrons are diffused. Since then, the field has continued to evolve as scientists work to bring thermoelectric to a scalable technology.
Joshua Zide, an associate professor of materials science and engineering at the University of Delaware, studies rare earth elements, particularly terbium, which is part of the chemical element combination for Saini’s discovery. He says that terbium isn’t necessarily as abundant as the researchers suggest although the amount used within the chemical composition may make large quantities a moot point.
“[Terbium] is, in fact, far more common than tellurium, which is commonly used in thermoelectric but is actually somewhat rare,” says Zide. “This has resulted in large price increases in recent years as demand has soared for both thermoelectric and CdTe solar [cadmium telluride photovoltaic solar cells—the second most common ones on the market].”
Saini says that this thermoelectric technology took nearly ten years to come to fruition, with the initial goal being to create an efficient material before the team added bio-friendly to its final requirements. Once the product is patented, they want to introduce it commercially. “At this point, we can only say that in cars there is a lot of waste heat, which can be used to convert into electricity,” says Saini.
The future of thermoelectric power is promising, especially with this new discovery. Art Gossard, professor emeritus of materials and electrical and computer engineering at the University of California-Santa Barbara, believes the new technology could have future applications in military advancement, particularly the all-electric ship.
“You could use the heat that came from your boilers and reactors to generate electricity that would then drive the electric motor and push the electric ship,” says Gossard. “This ship would have the advantage of not leaving a plume of hot water behind, which makes it easier to track. But it would require megawatts of power, and thermoelectric is not scaled up to that extent yet.”
With this material, perhaps we will get there.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/Kristen_A._Schmitt.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/Kristen_A._Schmitt.jpg)